What is 1,3-dipolar cycloaddition?
The 1,3-dipolar cycloaddition is a type of chemical reaction that utilizes 1,3-dipolar molecules, also known as 1,3-dipoles, and dipolarophiles, similar to how dienes and dienophiles are used in Diels-Alder cycloadditions. The pioneering work on this reaction was conducted by Huisgen in the 1960s, and subsequent studies were carried out by Huisgen, Padwa, Zecchi, and others.
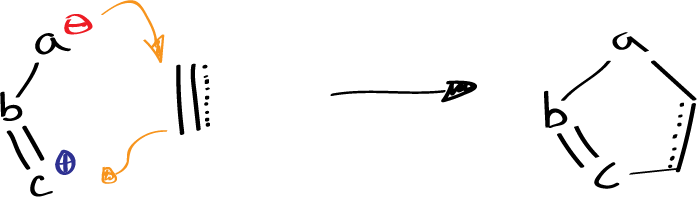
a, b, c = C, O, N
These 1,3-dipolar molecules are usually formed by the combination of carbon, nitrogen, and oxygen, and can be classified into two categories: the propargyl-allenyl type and the allyl type. The dipolarophiles can be either alkenes or alkynes. The end result of the reaction is a diverse array of five-membered heterocyclic molecules, which are frequently utilized in medicinal and pharmaceutical chemistry.
The use of the 1,3-dipolar cycloaddition is extensive in the formation of diverse five-membered heterocyclic molecules. This is achieved by combining various dipolar molecules, as previously mentioned, with either alkenes or alkynes.
References
- Huisgen, R., Fleischmann, R., and Eckell, A. (1960). Azomethin-imine, eine neue klasse zwitterionischer verbindungen. [Azomethine-imines, a new class of zwitterionic compounds] Tetrahedron Letters, 1(33), 1-4. https://doi.org/10.1016/S0040-4039(01)99303-7
- Huisgen, R., and Eckell, A. (1960). 1,3-Dipolare additionen der azomethin-imine. [1,3-Dipolare Additionen der Azomethin-imine] Tetrahedron Letters, 1(33), 5-8. https://doi.org/10.1016/S0040-4039(01)99304-9
- Grashey, R., Huisgen, R., and Leitermann, H. (1960). 1,3-Dipolare additionen der nitrone. [1,3-Dipolar additions of nitrones] Tetrahedron Letters, 1(33), 9-13. https://doi.org/10.1016/S0040-4039(01)99305-0
- 1,3-Dipolar cycloadditions. 76. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates
Rolf Huisgen
The Journal of Organic Chemistry 1976 41 (3), 403-419
DOI: 10.1021/jo00865a001 - Huisgen, R., Seidel, M., Wallbillich, G., and Knupfer, H. (1962). Diphenyl-nitrilimin und seine 1.3-dipolaren additionen an alkene und alkine. Tetrahedron, 17(1–2), 3-29. https://doi.org/10.1016/S0040-4020(01)99001-5
- Huisgen, R. (1963), 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl., 2: 565-598. https://doi.org/10.1002/anie.196305651
- Huisgen, R. (1963), Kinetics and Mechanism of 1,3-Dipolar Cycloadditions. Angew. Chem. Int. Ed. Engl., 2: 633-645. https://doi.org/10.1002/anie.196306331
- Huisgen, R., “1,3-Dipolar Cycloadditions – Introduction, Survey, Mechanism” in 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, ed. Padwa, A. [General Heterocyclic Chemistry Series, ed. Talyor, E. C. and Weissgberger, A.], John Wiley & Sons, New York, 1984, pp. 1–176.