What is Arndt-Eistert synthesis?
The Arndt-Eistert synthesis, also known as the Arndt-Eistert acid synthesis or Arndt-Eistert reaction, is a chemical reaction that was first reported by Arndt (Istanbul University) and Eistert (BASF) in 1935.
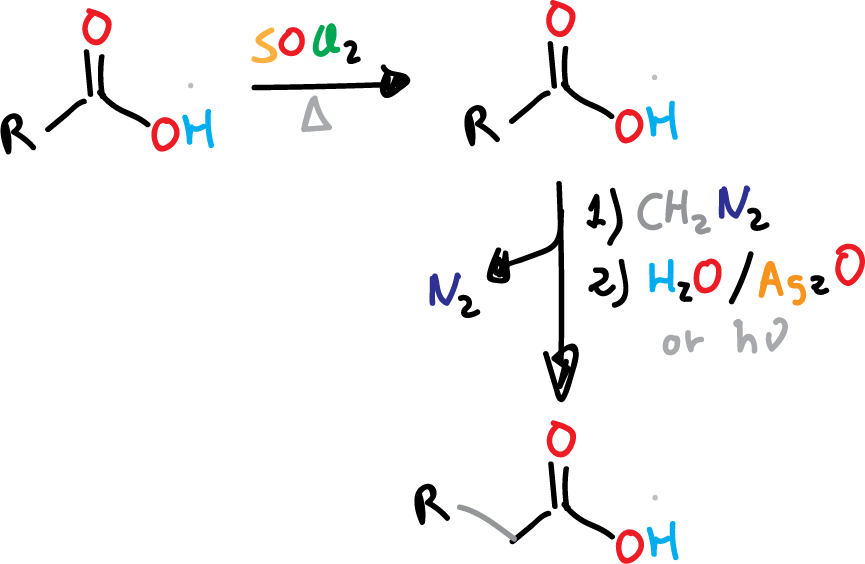
The reaction involves the extension of a carboxylic acid by one CH2 unit through the reaction of acyl chloride with diazomethane CH2NH2. The Arndt-Eistert homologation has been extensively studied and is a well-established method for carboxylic acid synthesis. In addition to diazomethane, other reagents have also been used to achieve similar results in extending the length of carboxylic acids.
One of the key features of the Arndt-Eistert synthesis is that it allows for the synthesis of carboxylic acids and carboxylic acid derivatives that are not easily accessible through other methods. This makes it a valuable tool in the synthesis of complex molecules and in the development of new pharmaceuticals and other compounds..
There are several variations of the Arndt-Eistert synthesis, including the use of different nucleophiles and the introduction of additional substituents onto the intermediate. These variations allow for the synthesis of a wider range of compounds and can be useful in the synthesis of more complex molecules..
Summary
The Arndt-Eistert synthesis is a valuable tool in the synthesis of carboxylic acids and carboxylic acid derivatives, and it has a number of applications in the synthesis of complex molecules and in the development of new compounds..
Example
Here is an example of an Arndt-Eistert synthesis:
Starting material: Ethyl diazoacetate
Nucleophile: Ethanol
Product: Ethyl acetate
The reaction is carried out by mixing ethyl diazoacetate and ethanol in a suitable solvent. The mixture is then heated to promote the reaction..
The mechanism of the reaction is not fully understood, but it is believed to involve the formation of a cyclic intermediate that undergoes further rearrangements to give the desired product. The intermediate may be formed through a concerted process, in which both the ethyl diazoacetate and the ethanol react simultaneously to form the cyclic species. Alternatively, the intermediate may be formed through a stepwise process, in which the ethyl diazoacetate adds to the ethanol in a first step, followed by a second step in which the intermediate cyclizes..
Once the intermediate has been formed, it undergoes further rearrangements to give the final product, ethyl acetate..
Overall, this example illustrates the use of the Arndt-Eistert synthesis to synthesize an ester from a diazo compound starting material and a nucleophile..
Mechanism of reaction
The mechanism of the Arndt-Eistert synthesis is not fully understood, but it is believed to involve the formation of a cyclic intermediate that undergoes further rearrangements to give the desired product. The exact mechanism may vary depending on the specific conditions and reagents used in the reaction..
Here is a general outline of the possible mechanisms of the Arndt-Eistert synthesis:
Formation of the cyclic intermediate: The diazo compound adds to the nucleophile to form a cyclic intermediate. This step may occur through a concerted process, in which both the diazo compound and the nucleophile react simultaneously to form the cyclic species, or through a stepwise process, in which the diazo compound adds to the nucleophile in a first step, followed by a second step in which the intermediate cyclizes..
Rearrangement of the intermediate: The cyclic intermediate may undergo further rearrangements to give the desired product. These rearrangements may involve the shifting of substituents or the formation and breaking of bonds within the intermediate..
Formation of the final product: The rearranged intermediate is converted to the desired product, which may be a carboxylic acid or a carboxylic acid derivative, depending on the specific conditions and reagents used in the reaction..
Overall, the exact mechanism of the Arndt-Eistert synthesis is not fully understood and may vary depending on the specific conditions and reagents used in the reaction. However, the reaction is believed to involve the formation of a cyclic intermediate that undergoes further rearrangements to give the desired product.
References
Arndt, F. and Eistert, B. (1935), Ein Verfahren zur Überführung von Carbonsäuren in ihre höheren Homologen bzw. deren Derivate. [A process for converting carboxylic acids into their higher homologues or their derivatives.] Ber. dtsch. Chem. Ges. A/B, 68: 200-208. https://doi.org/10.1002/cber.19350680142