What is Fischer indole synthesis?
Fischer indole synthesis, also known as the Fischer indolization, is an organic chemistry reaction that allows for the synthesis of indoles, a class of aromatic heterocyclic compounds that are found in a variety of natural products and drugs. The reaction was developed by Emil Fischer in 1883 and is named after him..
Fischer indole synthesis involves the reaction of an aryl aldehyde or an aryl ketone with aniline or a substituted aniline in the presence of a concentrated sulfuric acid catalyst. The reaction proceeds through an electrophilic substitution mechanism, with the aniline serving as the nucleophile and the aryl aldehyde or aryl ketone serving as the electrophile..
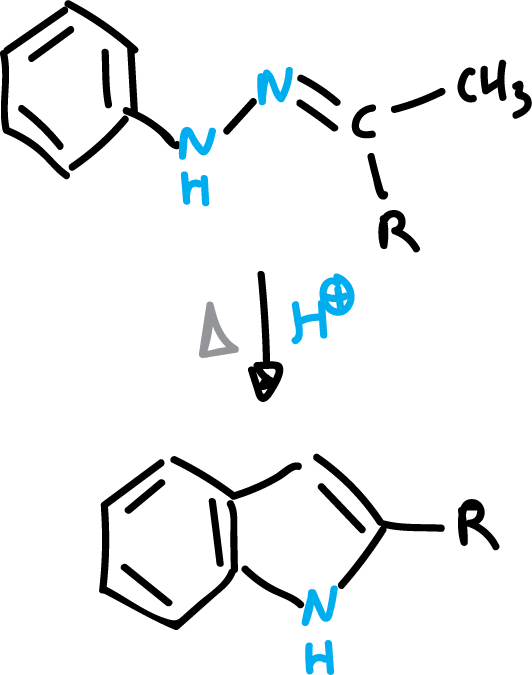
The Fischer synthesis is the most widely used method of obtaining indole. It consists of the transposition of a phenylhydrazone of the ortho-benzylene type, which cycles with loss of ammonia. The reaction is catalyzed by hydrochloric acid (HCl).
Fischer indole synthesis has several advantages over other methods for the synthesis of indoles. It is relatively simple and can be easily scaled up for large-scale synthesis. It also has a high yield and good selectivity, with little formation of byproducts..
Fischer indole synthesis has been used in the synthesis of a variety of natural products and drugs, including the anti-inflammatory drug indomethacin and the anti-cancer agent doxorubicin. It has also been used in the synthesis of other heterocyclic compounds and in the preparation of dyes and pigments..
Summary
Fischer indole synthesis is an important tool in the field of organic chemistry due to its ability to efficiently synthesize indoles, which have a wide range of applications in the synthesis of drugs and other organic compounds..
Example
The Fischer indole synthesis is a chemical reaction that produces indole derivatives from a substituted aniline and a carboxylic acid. An example of a Fischer indole synthesis is the reaction between aniline and acetic acid:
The aniline is treated with a strong acid catalyst, such as sulfuric acid, which protonates the nitrogen atom, making it more electrophilic..
The carboxylic acid is then added, and the carboxyl group attacks the electrophilic nitrogen atom, forming an intermediate called an anilide..
The anilide intermediate then loses a molecule of water, forming an imine, also called a Schiffs base..
The imine is then heated, which causes a cyclization reaction forming an indole ring..
The acid catalyst is then neutralized to give the final product..
The Fischer indole synthesis is a useful synthetic tool for the preparation of a wide variety of indole derivatives which are found in many biologically active compounds such as neurotransmitters and alkaloids..
Mechanism of reaction
The Fischer indole synthesis is a chemical reaction that converts an arylhydrazine and an aldehyde or ketone to an indole. The mechanism of the reaction is as follows:
The reaction mechanism starts with a protonation.
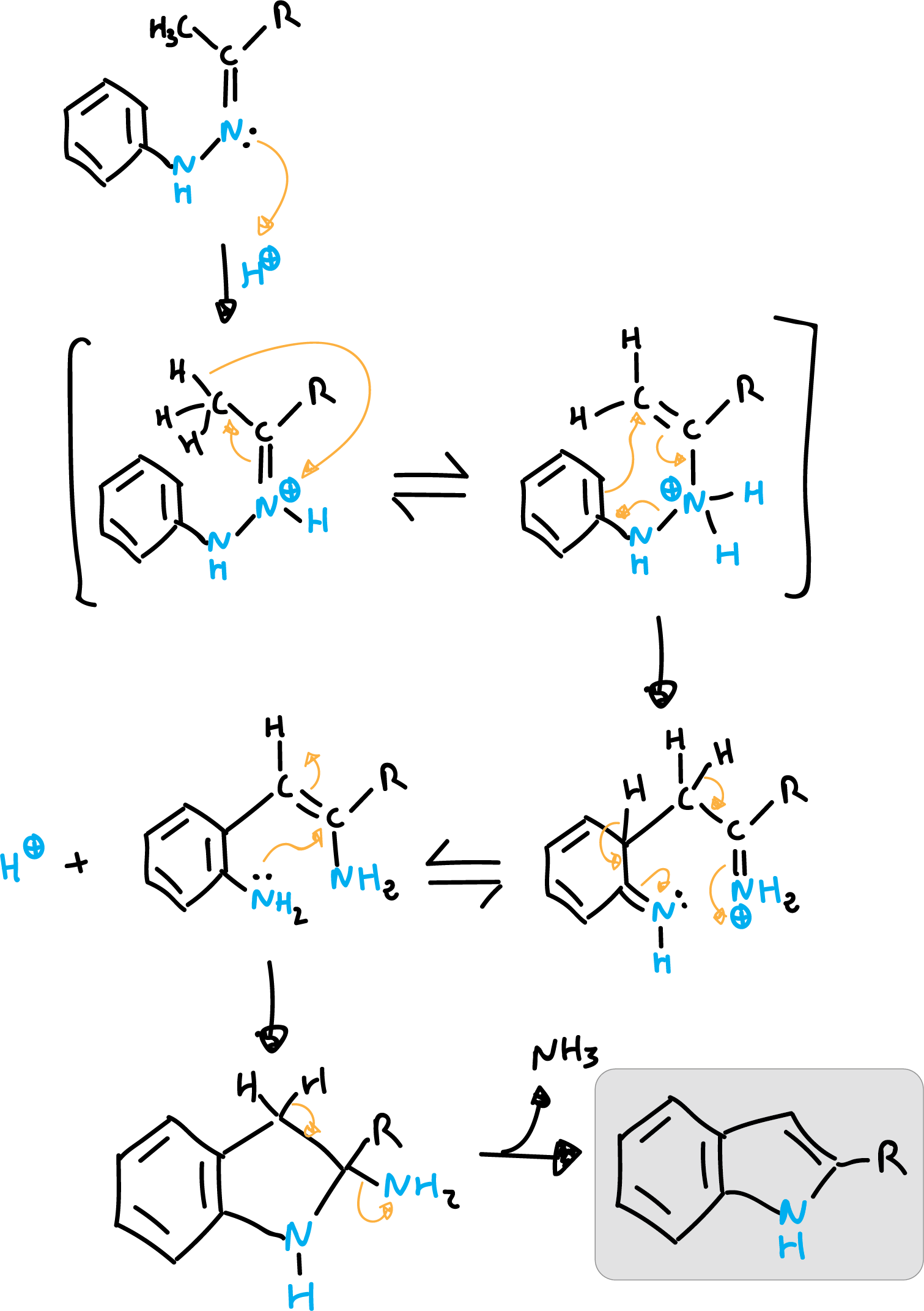
- Step 1: An arylhydrazine molecule reacts with an aldehyde or ketone in the presence of an acid catalyst to form an arylhydrazone intermediate..
- Step 2: The arylhydrazone intermediate then undergoes a tautomerization process, in which the nitrogen atom shifts its position, to form an imine intermediate..
- Step 3: The imine intermediate then loses water to form the final indole product..
- Step 4: The reaction is acid catalyzed, the acid acts as protonating agent for the nitrogen of the hydrazine in step 1, and deprotonating agent for the nitrogen of the imine in step 3..
References
- Fischer, E. and Jourdan, F. (1883), Ueber die Hydrazine der Brenztraubensäure. [On the hydrazines of pyruvic acid.] Ber. Dtsch. Chem. Ges., 16: 2241-2245. https://doi.org/10.1002/cber.188301602141
- Fischer, E. and Hess, O. (1884), Synthese von Indolderivaten. [Synthesis of indole derivatives.] Ber. Dtsch. Chem. Ges., 17: 559-568. https://doi.org/10.1002/cber.188401701155