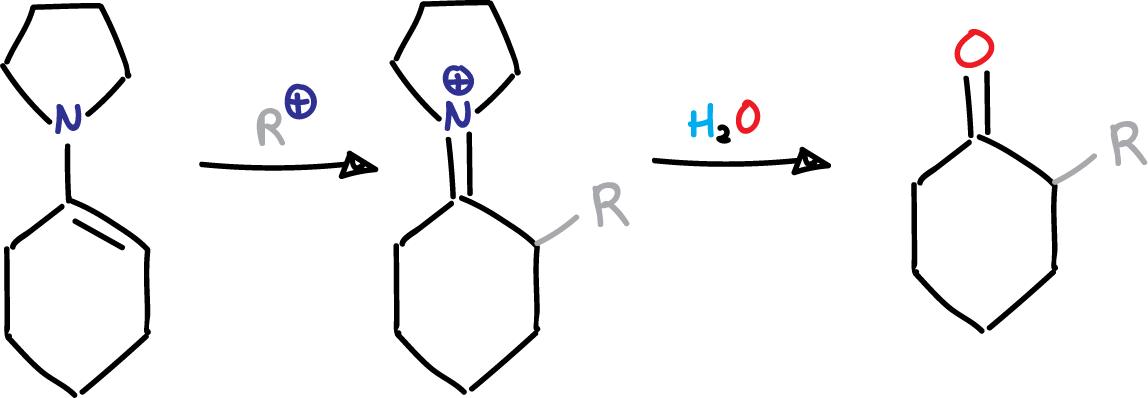
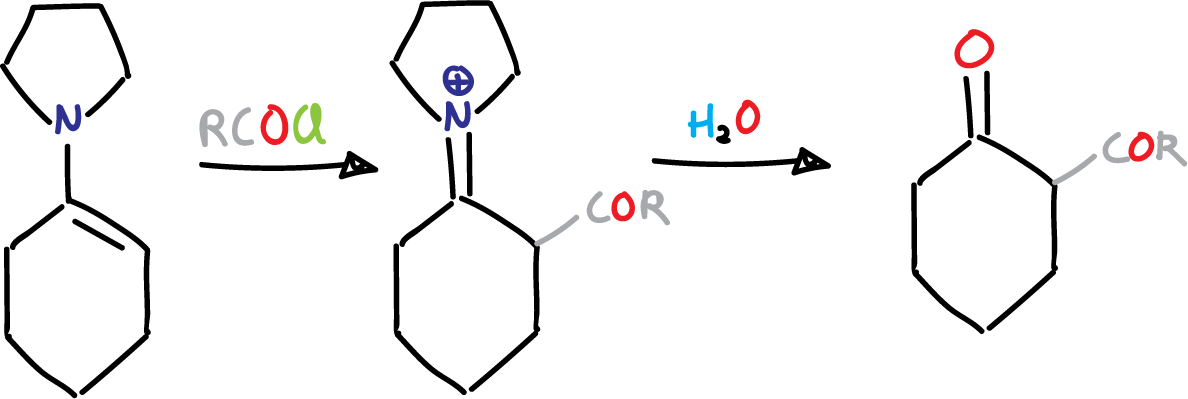
What is Stork enamine reaction?
The Stork enamine reaction is a method for synthesizing α-alkyl or α-acyl carbonyl compounds from enamines and alkyl or acyl halides. The discoverer of this reaction, Gilbert Stork, was the Eugene Higgins Professor of Chemistry Emeritus at Columbia University.
This reaction, also known as the Stork enamine alkylation reaction and Stork enamine acylation reaction, gives moderate yields when using simple alkyl halides. However, when more electrophilic reactants such as allylic, benzylic, or propargylic halides, or α-halo ethers, α-halo esters, or α-halo ketones are used, the yields can be improved for the alkylated product.
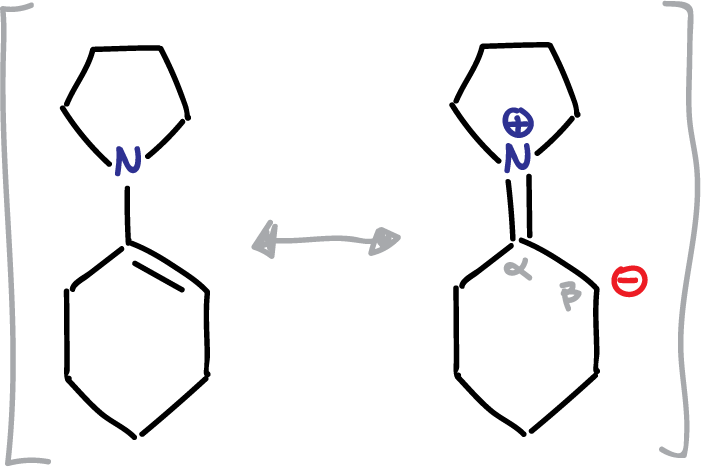
Enamines play a crucial role as intermediates in organic synthesis. This is due to the nucleophilic nature of the β-carbon atom, as depicted in the resonance structures shown in the figure. The reactivity of enamines arises from the presence of the negative charge on the β-carbon atom.
Enamines that correspond to ketones or aldehydes can be obtained by using p-toluenesulfonic acid (TsOH) in combination with secondary amines, as illustrated in the figure. Cyclic secondary amines, such as piperidine, pyrrolidine, and morpholine, are commonly utilized in this process.
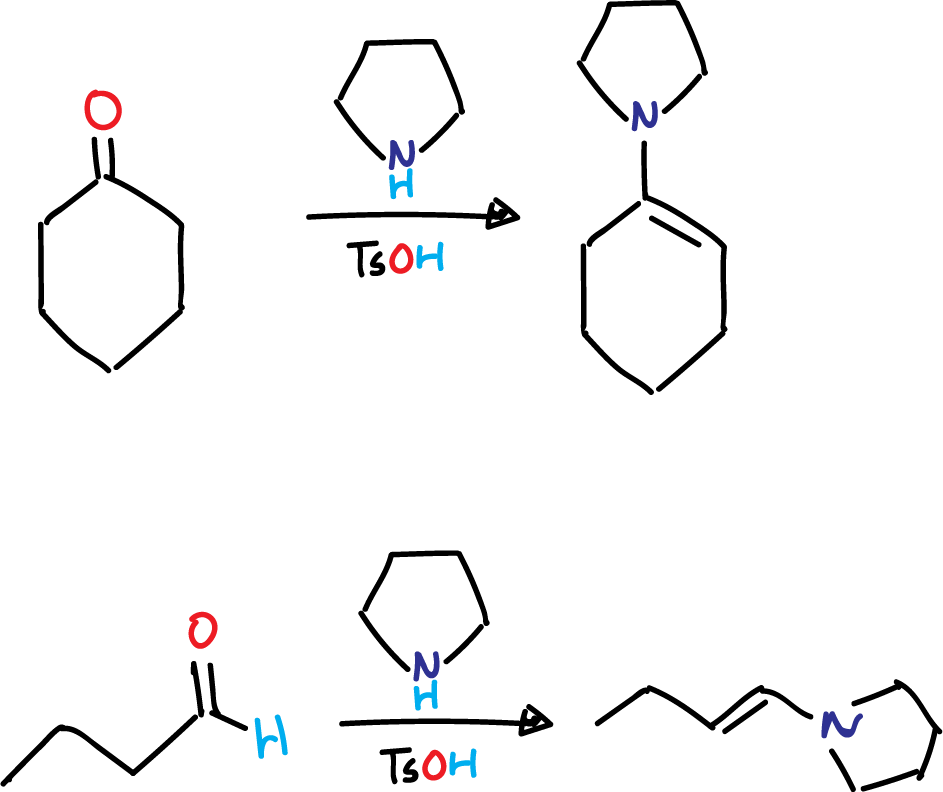
In order to shift the equilibrium, it is common practice to eliminate the water produced during the enamine formation reaction through azeotropic distillation with either benzene or toluene.
References
- A New Synthesis of 2-Alkyl and 2-Acyl Ketones
Gilbert Stork, Ross Terrell, and Jacob Szmuszkovicz
Journal of the American Chemical Society 1954 76 (7), 2029-2030
DOI: 10.1021/ja01636a103 - A New Alkylation of Carbonyl compounds. II1
Gilbert Stork and Hans K. Landesman
Journal of the American Chemical Society 1956 78 (19), 5128-5129
DOI: 10.1021/ja01600a087