What is Wolff-Kishner reduction?
The transformation of carbonyl compounds into hydrocarbons through the decomposition of hydrazone intermediates is known as the Wolff-Kishner reduction. The reaction was first reported in 1911 by Kishner (Tomsk Polytechnic University) and almost concurrently in 1912 by Wolff (University of Jena). However, both methods are impractical for laboratory transformation due to inconvenient procedures.
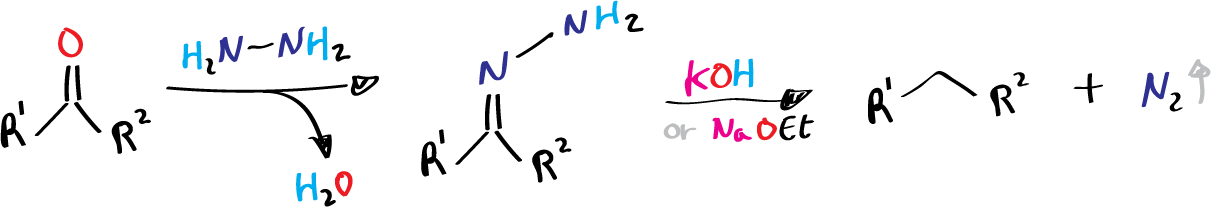
- R1 = alkyl, aryl
- R2 = H, alkyl, aryl
Several modifications have been reported, with the Huang-Minlon procedure being the most popular. This modification involves heating carbonyl compounds, hydrazine hydrate, and a base with diethylene glycol (DEG). The reaction rate is reasonable only when the temperature is raised above 190 ºC.
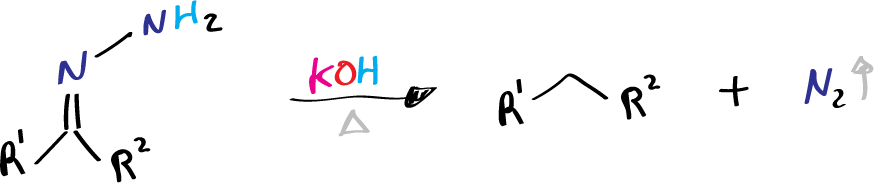
- R1 = alkyl, aryl
- R2 = H, alkyl, aryl
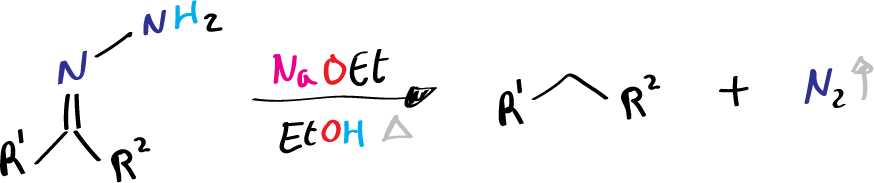
- R1 = alkyl, aryl
- R2 = H, alkyl, aryl
The Wolff-Kishner reduction is especially valuable for large-scale reduction of carbonyl compounds. However, when the concentration of reactant is ≥ 0.2 mol %, the presence of impurity is detectable. One of the side reactions is Kishner-Leonard elimination, which often occurs for the hydrazone substrates with an α-heteroatomic substituent.
The reaction has been successfully used for strained cyclopropyl ketones, as well as 1,3-cyclobutanediones.
The Huang-Minlon reduction of 2-acyl-1,3-indandiones affords 1,4-dihydro-3-substituted-indeno[1,2-c]pyrazoles. Various thiophene derivatives can also be decomposed at relatively low temperatures, such as in the range between 90 and 140 ºC.
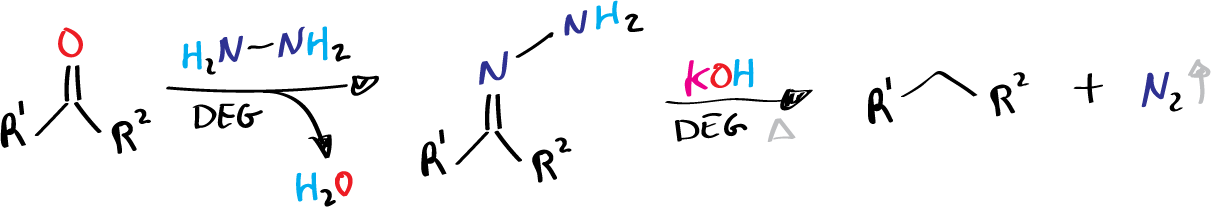
- R1 = alkyl, aryl
- R2 = H, alkyl, aryl
- DEG = diethylene glycol (see list of acronyms)
The Wolff–Kishner reduction is a useful method for the reduction of a wide range of carbonyl compounds, including aldehydes, ketones, and other functional groups. However, it has several limitations, including the need for high temperatures and the use of toxic hydrazine. As a result, it has largely been superseded by other reduction methods, such as the Clemmensen reduction ..
It’s worth noting that the Wolff-Kishner reduction is not limited to the reduction of acetaldehyde, it can be applied to other aldehyde and ketones, and it is also a useful method for the synthesis of alkanes, as well as for the reduction of functional groups..
The versatility of Wolff-Kishner reduction makes it highly useful in organic synthesis, particularly in the synthesis of multiwalled carbon nanotubes (MWNTs).
Mechanism of reaction
The mechanism of the Wolff-Kishner reduction involves the conversion of the carbonyl compound into a hydrazone intermediate, which is then converted into an alkane through a series of steps. The hydrazone intermediate is formed by the addition of hydrazine to the carbonyl compound, and this intermediate is then converted into an alkane by the addition of a reducing agent, such as sodium hydroxide.
It is generally agreed that the Wolff-Kishner reduction involves the formation of hydrazone in a manner analogous to the formation of imine between a carbonyl compound and a primary amine. Successive deprotonation on hydrazone eventually results in the evolution of nitrogen and the formation of hydrocarbon.
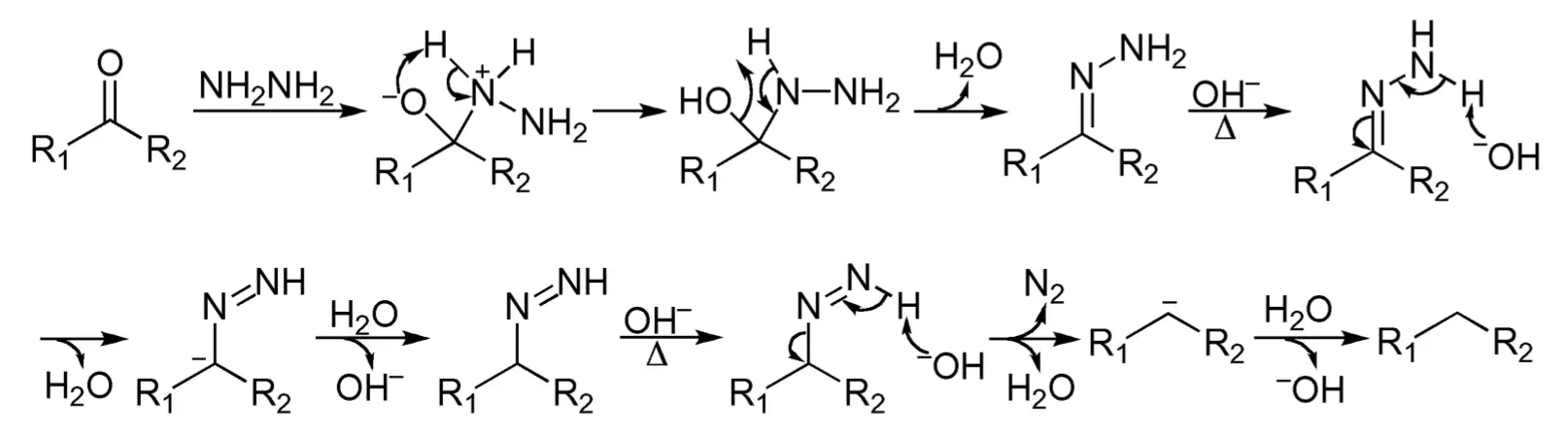
The mechanism of the Wolff-Kishner reduction can be divided into the following steps:
- The first step is the formation of a hydrazone intermediate, which is formed by the addition of hydrazine to the carbonyl compound. In this step, the nitrogen atom of the hydrazine molecule acts as a nucleophile, attacking the carbonyl carbon atom of the aldehyde or ketone.
- In the second step, the hydrazone intermediate is converted into a semicarbazone intermediate by the addition of a reducing agent, such as sodium hydroxide. In this step, the hydroxide anion acts as a nucleophile, attacking the nitrogen atom of the hydrazone intermediate. This results in the formation of a semicarbazone intermediate, which has a double bond between the nitrogen atom and the carbonyl carbon atom.
- The final step is the elimination of the nitrogen atom and the formation of an alkane. In this step, the nitrogen atom is eliminated by the loss of water, resulting in the formation of an alkane molecule. This step is often assisted by heating the reaction mixture.
It’s worth noting that the mechanism is a simplified version and that the actual reaction is more complex, involving the intervention of intermediates and the presence of other species in solution. Also, depending on the specific structure of the carbonyl compound and the choice of base and reducing agent, the intermediate and final products may vary.
In summary, the Wolff-Kishner reduction the mechanism of the reaction involves the formation of a hydrazone intermediate, which is then converted into a semicarbazone intermediate, and finally the elimination of the nitrogen atom and the formation of an alkane.
References
- N. Kishner, J. Russ. Phys. Chem. Soc. 43, 582 (1911)
- N. Kishner, J. Russ. Phys. Chem. Soc. 6, 347 (1912)
- Wolff, L. (1912), Chemischen Institut der Universität Jena: Methode zum Ersatz des Sauerstoffatoms der Ketone und Aldehyde durch Wasserstoff. [Erste Abhandlung.]. [Chemical Institute of the University of Jena: Method for replacing the oxygen atom of ketones and aldehydes by hydrogen. [First paper.]] Justus Liebigs Ann. Chem., 394: 86-108. https://doi.org/10.1002/jlac.19123940107
- A Simple Modification of the Wolff-Kishner Reduction
Huang-Minlon
Journal of the American Chemical Society 1946 68 (12), 2487-2488
DOI: 10.1021/ja01216a013