Go to the page with the list of problems.
Alkanes – solutions to problems
Solution 1:
(a) There are only three isomers: n-pentane (I), 2-methylbutane (II) and 2,2-dimethylpropane (III).
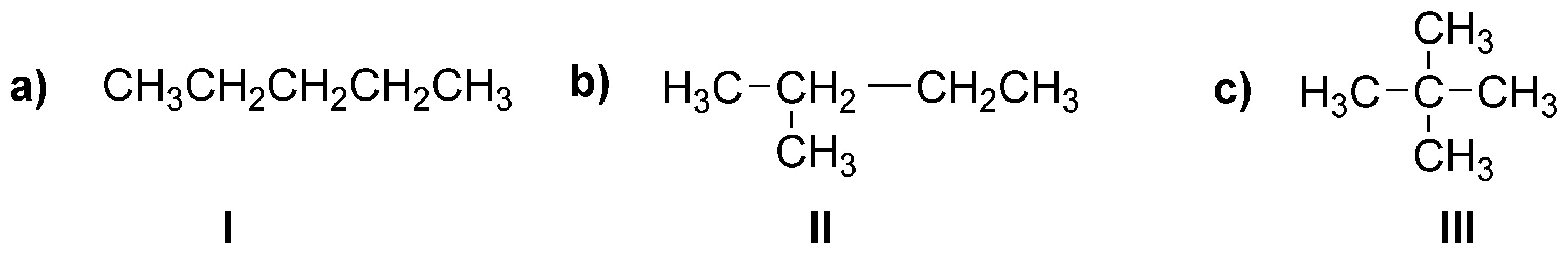
b) All methyl hydrogens (CH3) will be primary, methylene hydrogens (CH2) secondary and methylene hydrogens (CH) tertiary.
c) The only isomer that possesses only one type of hydrogens and therefore will give only one monobromination product will be 2,2-dimethylpropane (III).
Solution 2:
Solution 3:
Radical halogenation of alkanes and cycloalkanes depends on:
- The nature of the hydrocarbon
- The number of hydrogens of the same class
- The halogen used
For propane, the chlorination reaction is the result of 45% substitution of a hydrogen in the primary position and 55% substitution of a hydrogen in the secondary position, even though the ratio of primary to secondary hydrogens is 3:1 (propane has 6 primary hydrogens (2×(CH3-) and 2 secondary hydrogens (-CH2-)). Then, for chlorination, the relative reactivities of the hydrogens will be:
Primary reactivity = yield/no. of primary hydrogens = 45/6 = 7.5
Secondary reactivity = yield/no. of secondary hydrogens = 55/2 = 27.5.
Dividing by the smaller value:
Relative secondary/primary reactivity = 3.66/1.
For bromination, the major product is that from the substitution of a secondary hydrogen.Since the dissociation enthalpy for Cl2 and Br2 is similar (58 kcal/mol and 46 kcal/mol, respectively), the fundamental difference between the two reactions is in the process abstraction of the hydrogen atom. For bromination, therefore, the relative reactivities of the hydrogens will be:
Primary reactivity = yield/no. of primary hydrogens = 3/6 = 0.5
Secondary reactivity = yield/no. of secondary hydrogens = 97/2 = 48.5.
Dividing by the smaller value:
Relative secondary/primary reactivity = 97/1.
Proceeding in the same way for 2-methylpropane we determine the relative reactivities of the tertiary hydrogens with respect to the primary ones:
For chlorination:
Primary reactivity = yield/no. of primary hydrogens = 64/9 = 7.1
Tertiary reactivity = yield/no. of tertiary hydrogens = 36/1 = 36.0.
Dividing by the smaller value:
Relative tertiary/primary reactivity = 5.1/1.
For bromination:
Primary reactivity = yield/no. of primary hydrogens = 1/9 = 0.1
Tertiary reactivity = yield/no. of tertiary hydrogens = 99/1 = 99.0.
Dividing by the smaller value:
Relative tertiary/primary reactivity = 990/1.
The explanation is to be found in collision theory. When the X- radical is formed, the probability of collision with primary hydrogens is higher than with secondary hydrogens (statistical factor), but the activation energy for the formation of 1-halopropane is higher than that for the formation of 2-halopropane. Less energy is needed to form the secondary radical than the primary radical. Small differences in activation energy result in one reaction pathway predominating over another (energy factor).
On the other hand, although Cl- and Br- radicals have very different stability (Cl- is much less stable than Br-). Their tendency to collide effectively “with any” hydrogen offsets part of the energetic factor, thus increasing the ratio of substitution product in primary position.
Solution 4:
The monochlorination of 2,3-dimethylbutane is given by the following equation:
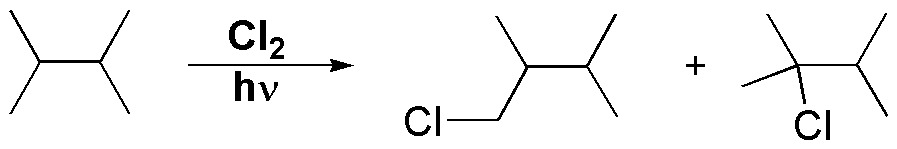
2,3-dimethylbutane has a total of 12 primary hydrogens and 2 tertiary hydrogens. The relative reactivity of each of the different types of hydrogens can be calculated from the chlorination data of 2-methylpropane. This molecule has the same type of hydrogens, with 9 primary hydrogens 3×(CH3-), and one tertiary hydrogen (>CH-). The reactivity corresponding to each hydrogen will be:
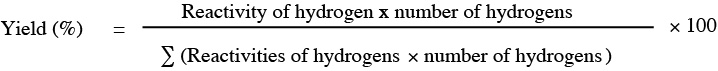
For 2,3-dimethylbutane the yield is obtained by applying the same formula, which for the formation of 1-chloro-2,3-dimethylbutane is:
yield (%) = [(1×12)/(1×12 + 5×2)] × 100 = 54.5%.
and for 2-chloro-2,3-dimethylbutane is:
yield (%) = [(5×2)/(1×12 + 5×2)] × 100 = 45.5%.
Solution 5:
For propane it will be in 2-bromopropane, for butane in 2-bromobutane, for 2-methylpropane 2-bromo-2-methylpropane, for cyclopentane Bromocyclopentane and for methylcyclopentane 1-bromo-1-methylcyclopentane.
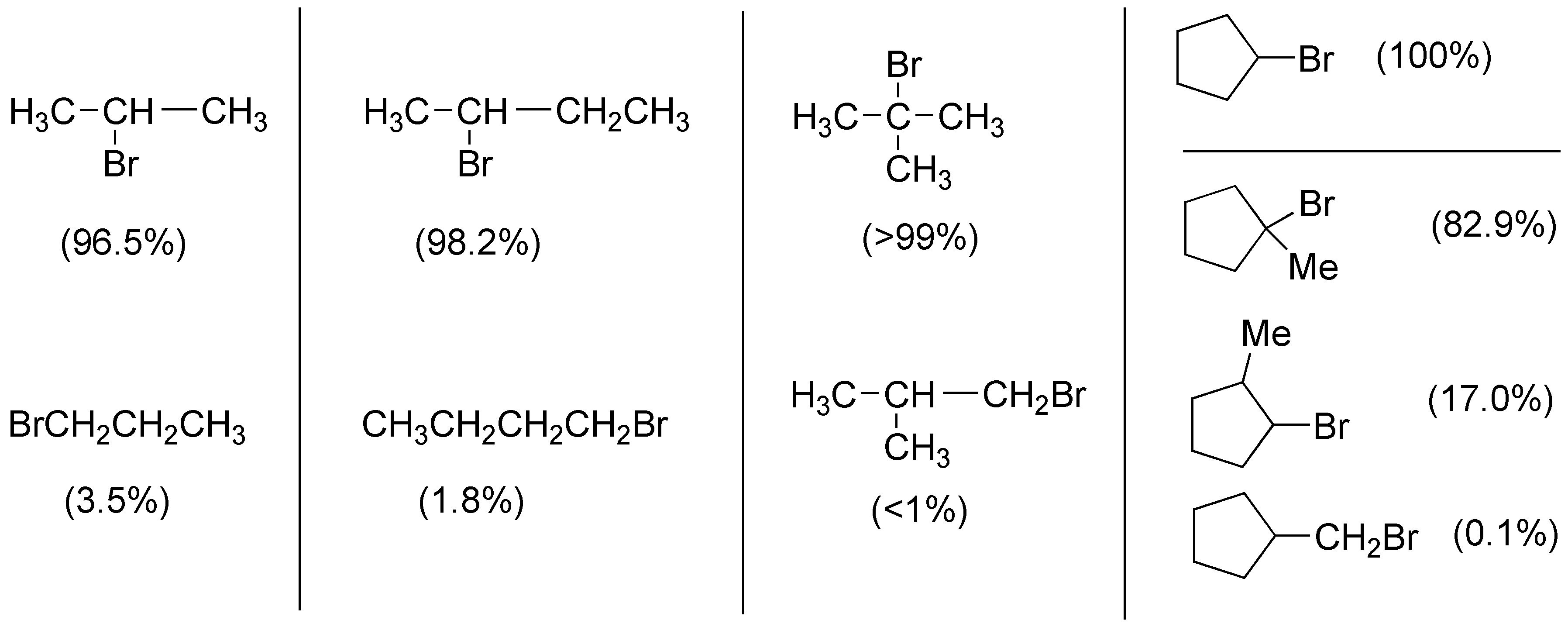
Solution 6:
I.- 3-methylpentane has 9 primary hydrogens, corresponding to the 3 methylenes of the 3×(CH3-) ends, 4 secondary hydrogens of the two methylenes -CH2– and 1 tertiary hydrogen in position 3 of the chain that gives rise to the most stable radical and therefore, the one that generates the substitution product.
II.- Ethylcyclohexane has 3 primary hydrogens, 12 secondary hydrogens and a single tertiary hydrogen, that of the carbon attached to the ethyl group, which will give rise to the substitution product.
III.- 2,3,4-trimethylpentane has 15 primary and 3 tertiary hydrogens, the latter of two types (with a 2:1 ratio), so the radical bromination reaction should give a mixture of about 66% of 2-bromo-2,3,4-trimethylpentane (IIIa) and 33% of 3-bromo-2,3,4-trimethylpentane (IIIb).
IV.- The compound 2,2,4-trimethylhexane has 15 primary hydrogens, 4 secondary hydrogens and a single tertiary hydrogen so the major product of the reaction will be 4-bromo-2,2,4-trimethylhexane.
V.- 1,1,2,3,3,3,4-hexamethylcyclopentane has two tertiary hydrogens at positions 2 and 4, two secondary hydrogens at the methylene at position 5 and 18 primary methyl hydrogens. The bromination reaction gives a mixture of 1-bromo-1,2,2,3,3,5,5-hexamethylcyclopentane and 1-bromo-1,2,2,3,4,4- hexamethylcyclopentane.
VI.- The alkane 2,2,3,3-tetramethylbutane has only 18 primary hydrogens, so the product of the monobromination of that compound will give 1-bromo-2,2,3,3-tetramethylbutane as the product.
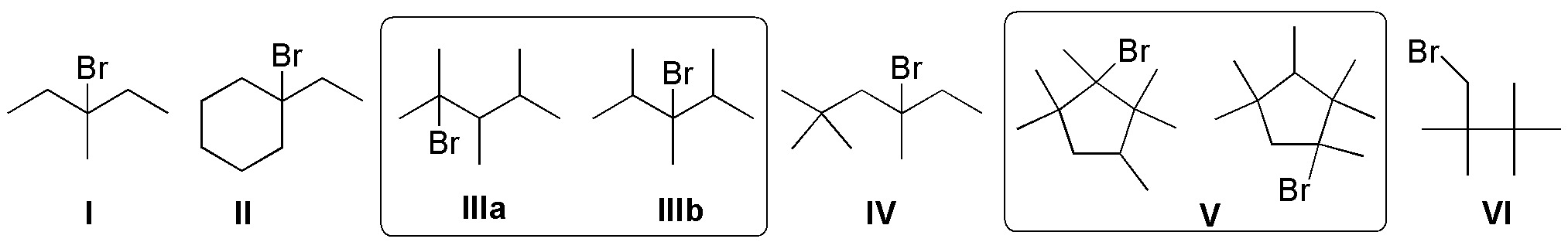
Solution 7:
2,4-dimethylpentane is a symmetric molecule, so the number of constitutional isomers is reduced.
![]()
The safest way to solve the problem is to place the chlorine atoms from left to right along the main chain, in principle not equivalent:
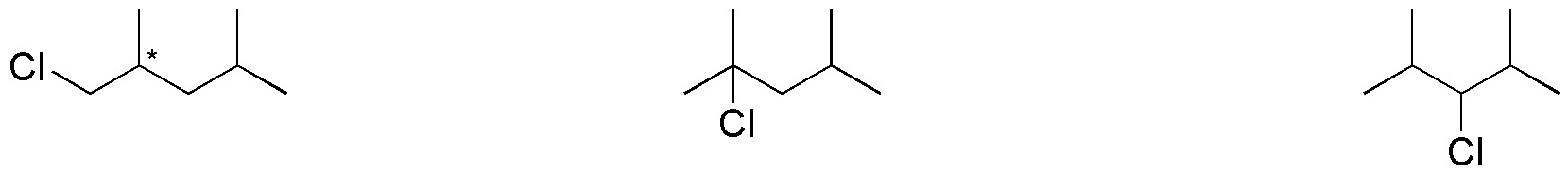
In this way, we obtain the 3 structures shown in the figure above: 1-chloro-2,4-dimethylpentane, 2-chloro-2,4-dimethylpentane and 3-chloro-2,4-dimethylpentane, respectively. Any other chlorine substitution is consistent with any of the above options.
Regarding the chirality of the products obtained, for 1-chloro-2,4-dimethylpentane, the carbon at position 2 (indicated by an asterisk) is attached to four different substituents (ClCH2-, (CH3)2CH2CH2-, CH3– y H-), it is therefore a chiral carbon. In the rest of the monochlorination products this circumstance does not occur, so it does not have chiral carbons.
Solution 8:
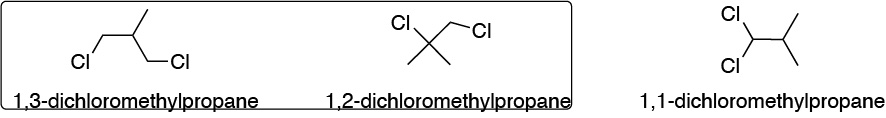
Methylpropane presents three methyls, with a total of 9 primary hydrogens and a tertiary hydrogen in position 2. The possibilities of substitution of two hydrogens simultaneously are:
- Substitution of two hydrogens attached to different carbons
- Substitution of two hydrogens attached to the same carbon
From the first possibility, the two structures in the box are deduced, and from the second option the 1,1-dichloromethylpropane.
Solution 9:
The bromination products will be three:
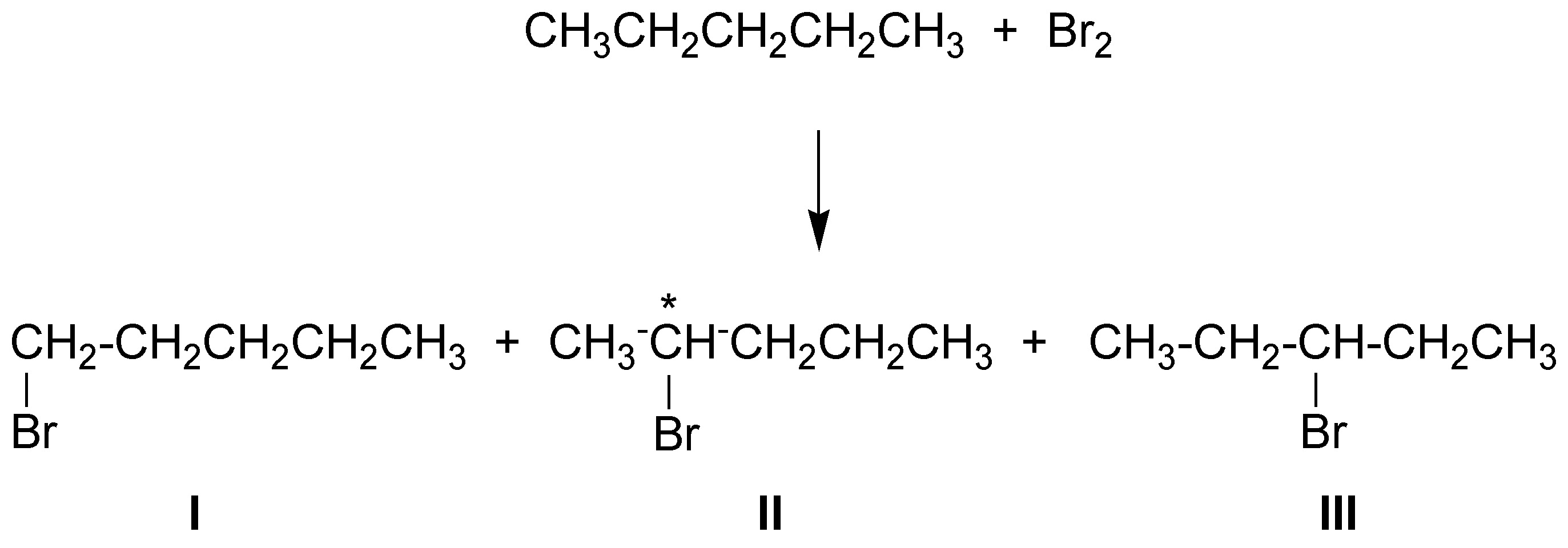
in the case of 2-bromopentane when an asymmetric carbon is created (the C-2) this will appear as racemic.
Solution 10:
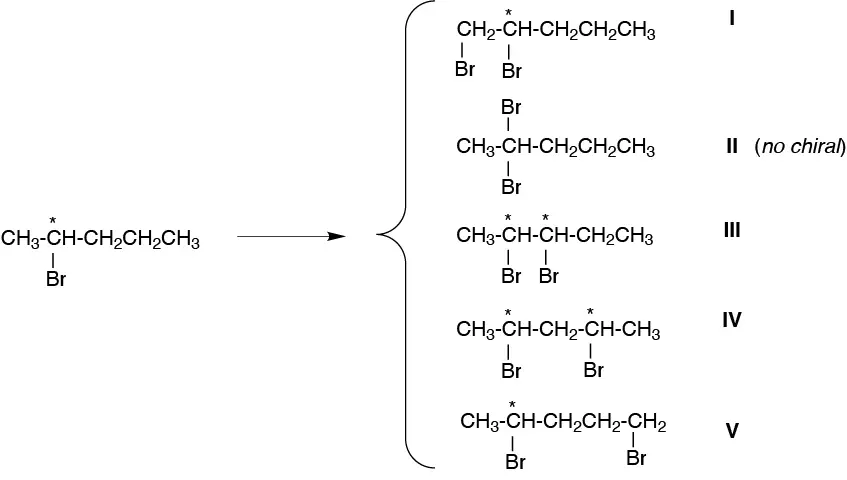
Taking into account the relative reactivity of the hydrogens, it will form:
II > III ≈ IV > I ≈ V
and as new asymmetric carbons have been created the above products will appear as:
I and V as pairs of enantiomers (racemic mixtures).
III as 4 stereoisomers.
IV as 3 stereoisomers.
Solution 11:
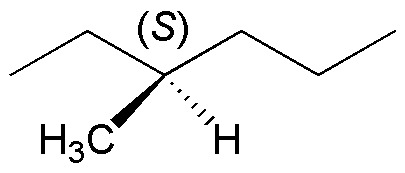
If the starting product is enantiomerically pure it will exhibit optical activity. The monobromination reaction leads mostly to the substitution product at position 3, since the radical generated at that position is tertiary and bromination, as explained above, is highly selective. The reason why an optically inactive reaction crude is produced is due to the structure of the radical intermediate. This is planar and the probability of attack by the bromine radical is the same on either side of the radical, thus resulting in racemization of the sample.
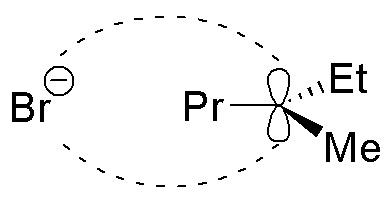
Solution 12:
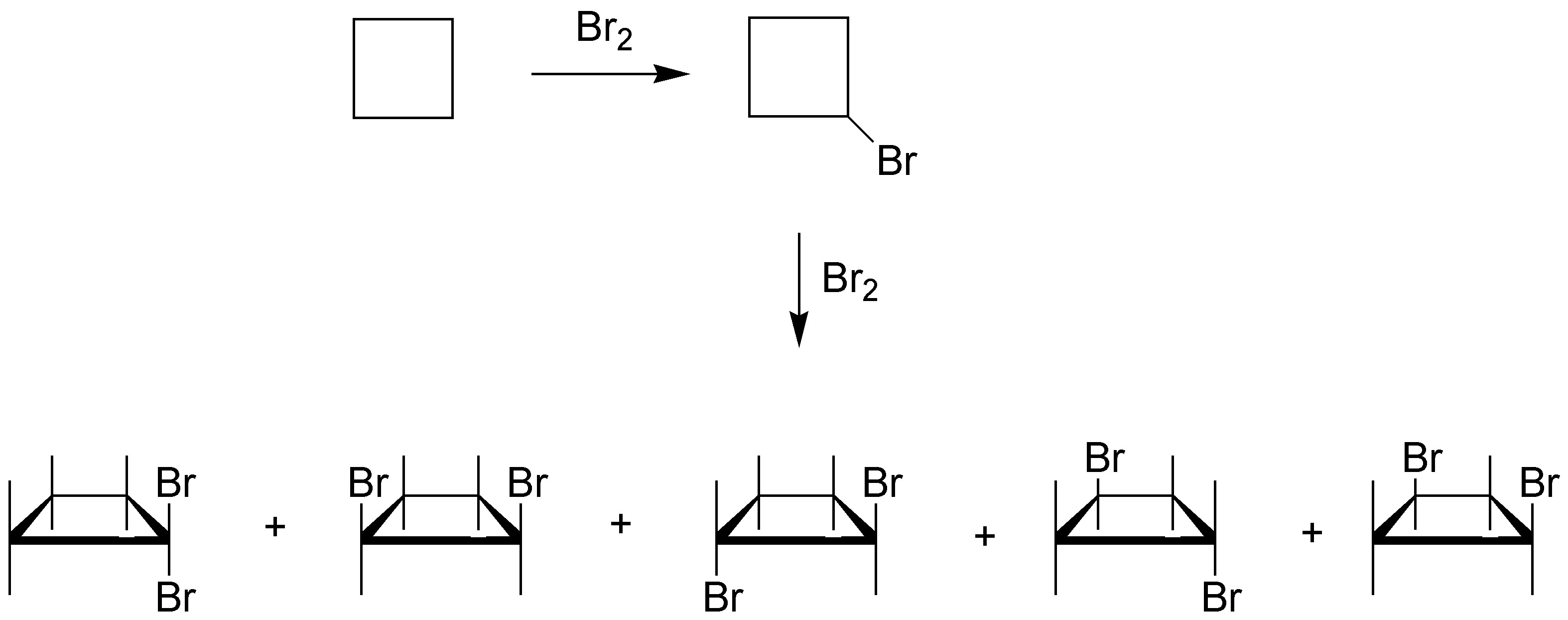
Solution 13:
Pyrolysis reactions on alkanes using various types of catalysts produce homolytic breaking of C-C bonds. The process is random, so all statistical possibilities are given:
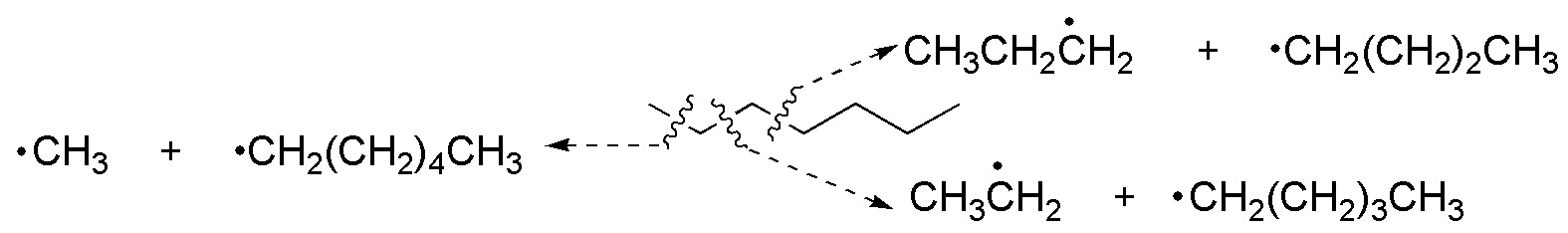
Methyl, ethyl, propyl, butyl, butyl, pentyl and hexyl radicals are obtained, which can combine with each other, to give ethane to dodecane. All possible combinations are listed in the following table.
From the industrial point of view, the most interesting combinations are those involving the formation of hydrocarbons of lower molecular mass, which is the procedure used to obtain gasoline from heavier petroleum fractions using the appropriate catalysts. The following table lists the possible combinations of the fragments obtained in the pyrolysis of heptane:
| methyl | ethyl | propyl | butyl | pentyl | hexyl | |
|---|---|---|---|---|---|---|
| methyl | ethane | |||||
| ethyl | propane | butane | ||||
| propyl | butane | pentane | hexane | |||
| butyl | pentane | hexane | heptane | octane | ||
| pentyl | hexane | heptane | octane | nonane | decane | |
| hexyl | heptane | octane | nonane | decane | undecane | dodecane |
In addition, from alkanes, alkenes are generated by hydrogen transfer from one radical to another and formation of a double bond:
R’-CH2-CH2· + R· → R’-CH=CH2 + R-H
Solution 14:
Complete combustion (oxidation) of n-pentane gives carbon dioxide and water. To adjust it, proceed as follows:
step-1) The number of carbons in CO2 must be the same as the number of carbons in C5H12 (n-pentane).
C5H12 + O2 → 5CO2 + H2O
step-2) The number of hydrogens in H2O must be the same as the number of hydrogens in C5H12
C5H12 + O2 → 5CO2 + 6H2O
step-3) The number of oxygens in CO2 and H2O (16) must be the same as that in O2
C5H12 + 😯2 → 5CO2 + 6H2O
Solution 15:
Proceeding the same as in the previous case.
step-1) The number of carbons in CO2 must be the same as the number of carbons in C6H14 (n-hexane).
C6H14 + O2 → 6CO2 + H2O
step-2) The number of hydrogens in H2O must be the same as the number of hydrogens in C6H14
C6H14 + O2 → 6CO2 + 7H2O
step-3) The number of oxygens in CO2 and H2O (19) must be the same as that in O2
C6H14 + 9.5O2 → 6CO2 + 7H2O
step-4) To avoid a fractional number multiply all terms by 2
2C6H14 + 19O2 → 12CO2 + 14H2O
Solution 16:
In incomplete combustion the product of the reaction is CO and water. We would proceed as in the previous cases:
step-1) The number of carbons in CO must be the same as the number of carbons in C8H18 (isooctane).
C8H18 + O2 → 8CO + H2O
step-2) The number of hydrogens in H2O must be the same as the number of hydrogens in C8H18 (isooctane)
C8H18 + O2 → 8CO + 9H2O
step-3) The number of oxygens in CO2 and H2O (17) must be the same as that in O2
C8H18 + 8.5O2 → 8CO + 9H2O
step-4) To avoid a fractional number all terms are multiplied by 2
2C8H18 + 17O2 → 16CO + 18H2O
Solution 17:
The following products would be formed:
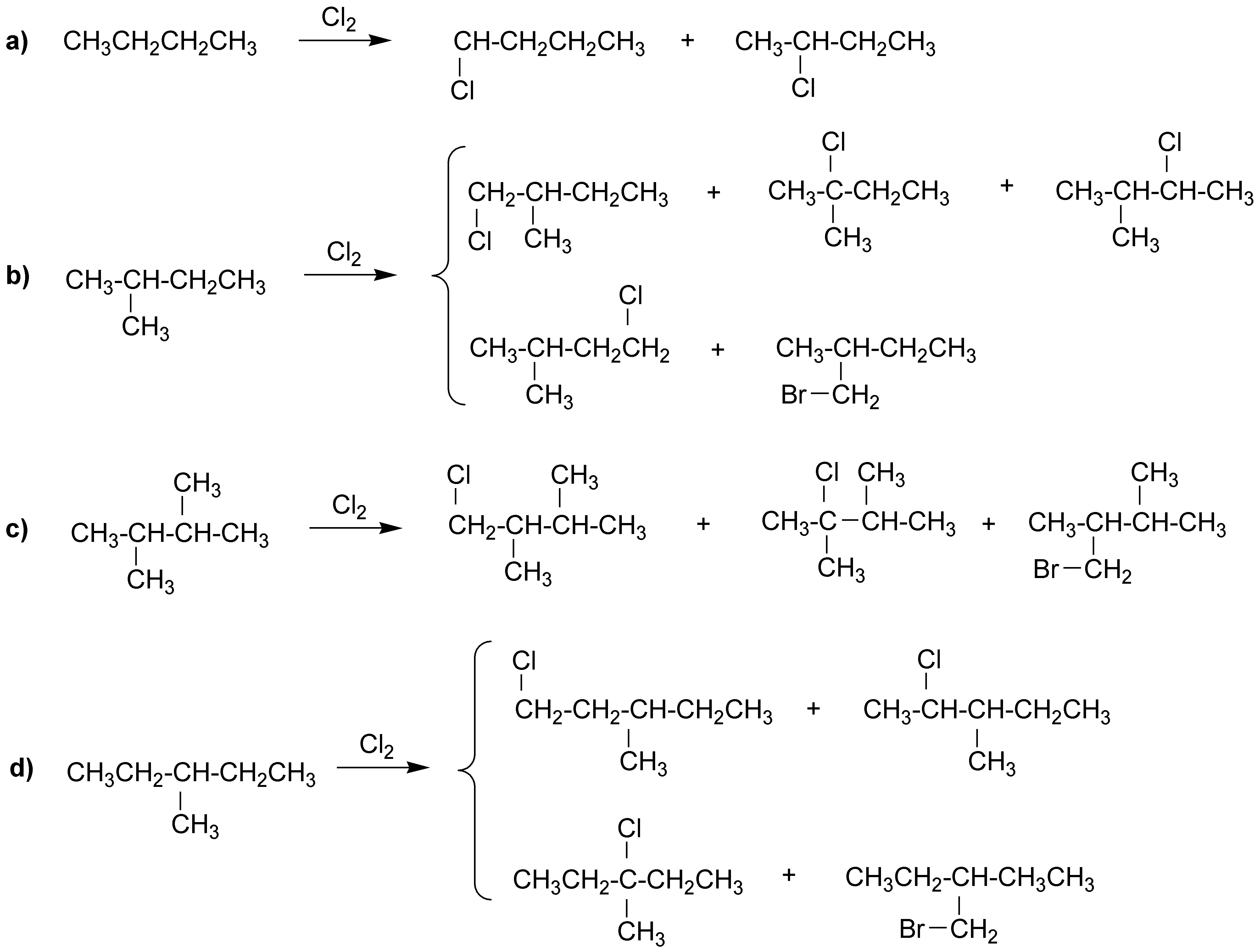
To calculate the proportions of each isomer we must take into account the relative reactivities 1º/2º/3º = 1/3.66/5.1. We would then have:
a) 1-chlorobutane: 29.07% and 2-chlorobutane: 70.93%.
b) 1-chloro-2-methylbutane: 28%; 2-chloro-2-methylbutane: 23.8%; 2-chloro-3-methylbutane: 34.2% and 1-chloro-3-methylbutane: 14%; 2-chloro-3-methylbutane: 34.2% and 1-chloro-3-methylbutane: 14%.
c) 1-Chloro-2,3-dimethylbutane: 54% and 2-Chloro-2,3-dimethylbutane: 46%.
d) 1-Chloro-3-methylpentane: 20.88%; 2-Chloro-3-methylpentane: 50.94%; 3-Chloro-3-methylpentane: 17.75% and 1-Chloro-3-ethylbutane: 10.44%.
Solution 18:
The following products would be formed:
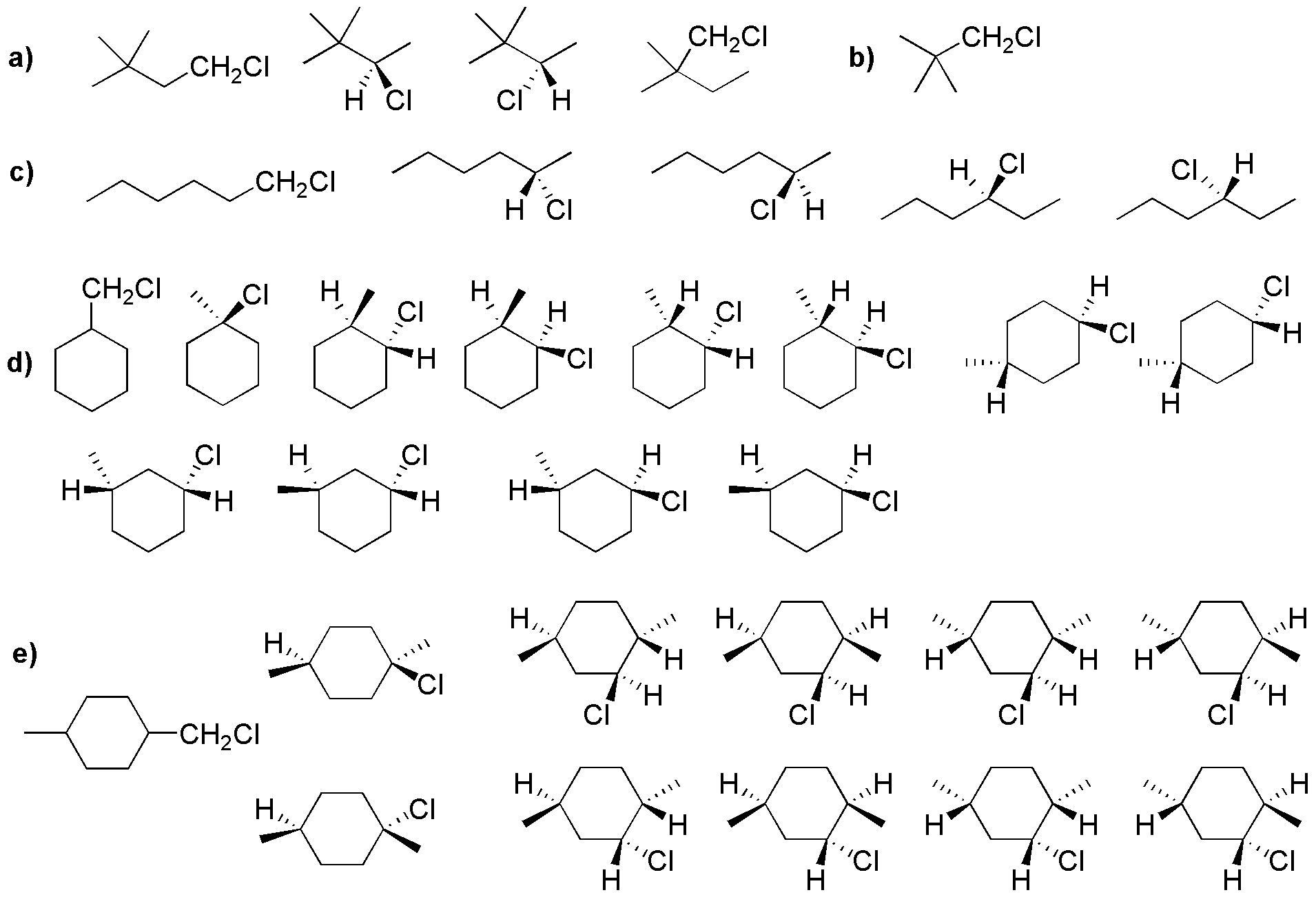
Solution 19:
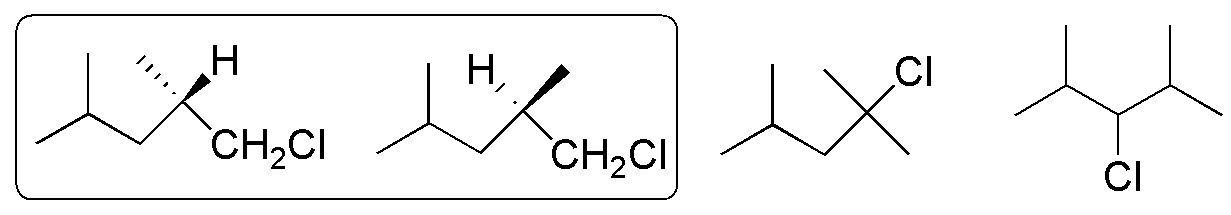
From the monochlorination of 2,4-dimethylpentane, the 4 products shown in the figure are obtained, the first two being enantiomers. However, the crude of the mixture would not present rotary optical power, since the same amount of R and S is obtained.
Solution 20:
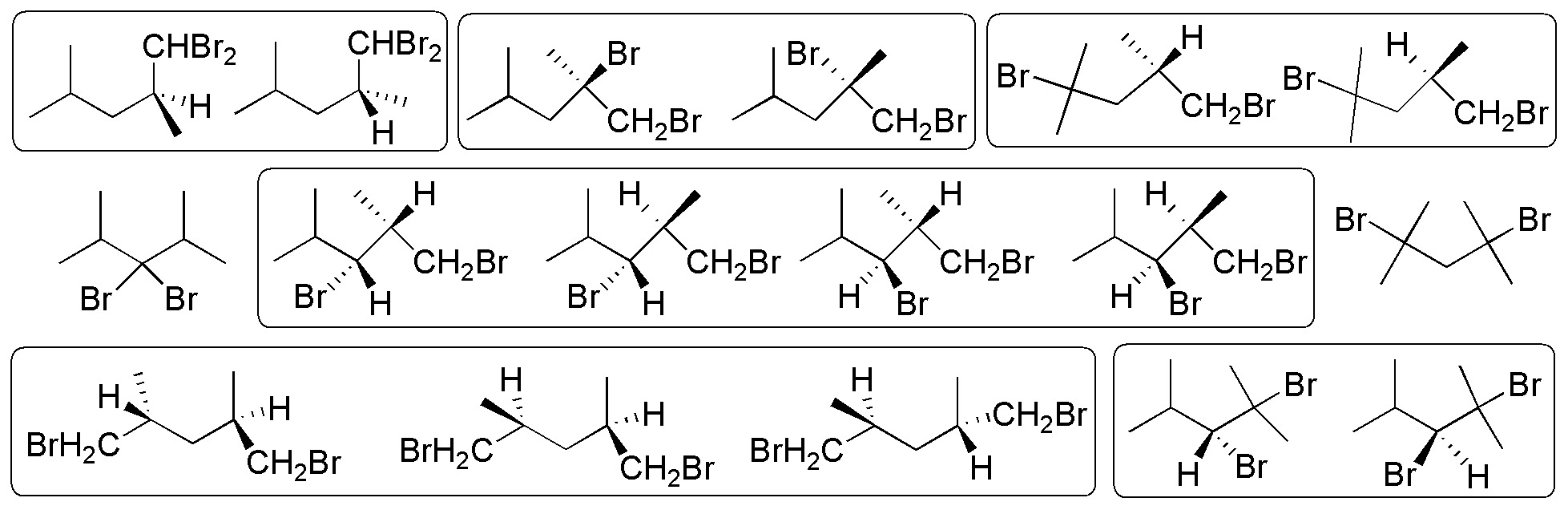
16 dibrominated products are obtained, of which the 15 highlighted by boxes present chiral carbons.
Solution 21:
Considering that in butane we have 6 primary and 4 secondary hydrogens the relative reactivities will be:
| Primary hydrogens | Secondary hydrogens | Reactivity of the hydrogens Relative secondary/primary reactivity | Relative reactivity secondary/primary |
|---|---|---|---|
| 6 |
4 primary = 28 / 6 = 4.6 secondary = 72 / 4 = 18 |
18 / 4.6 = 3.9 1 : 3.9 |
Solution 22:
Similarly as above, for 2-methylbutane we will have:
| Primary hydrogens
(CH3)2 |
Primary hydrogens
CH3 |
Secondary hydrogens CH2 | Tertiary hydrogens CH | Reactivity of hydrogens | Relative reactivity
3º : 2º : 1º : 1º |
|
|---|---|---|---|---|---|---|
| 6 | 3 | 2 | 1
primary (CH3)2– 27 / 6 = 4.5 primary CH3– 14 / 3 = 4.6 secondary -CH2– 36 / 2 = 18.0 tertiary -CH- 23 / 1 = 23.0 |
5.1 : 4 : 1 : 1 |
||
Solution 23:
For 2,2,4-trimethylpentane the products obtained and their ratio will be:
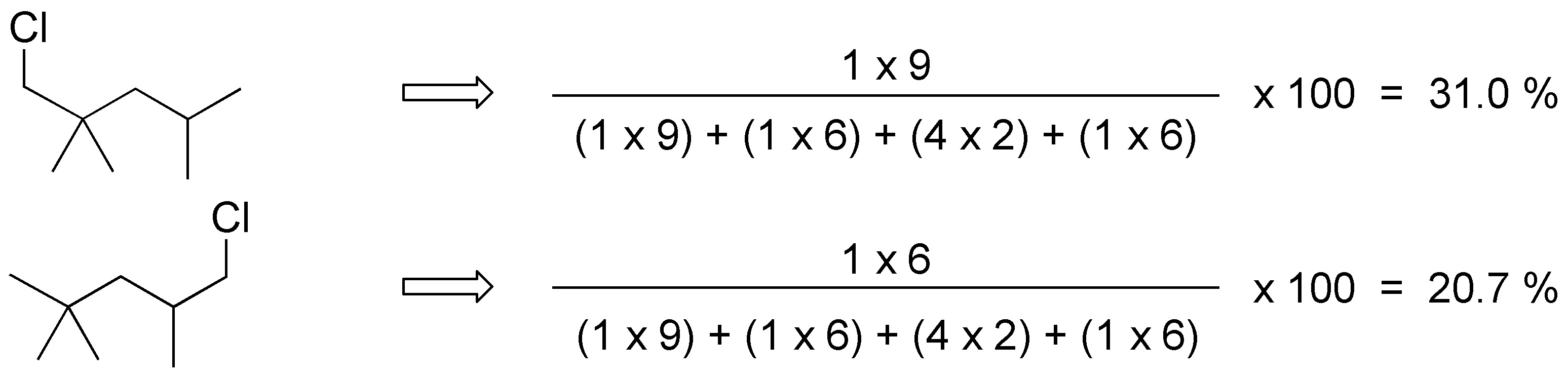
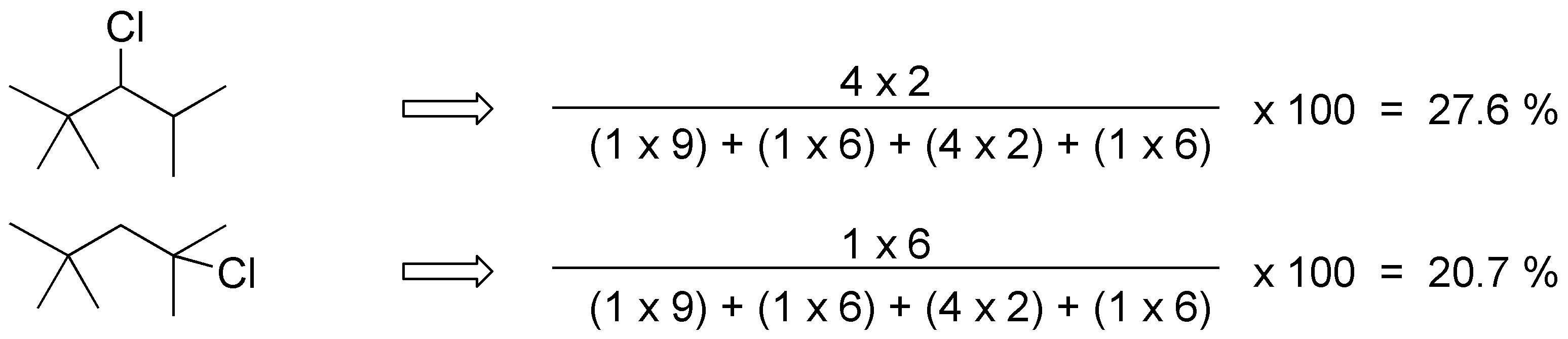
Solution 24:
Taking into account the relative reactivities the majority products will be:
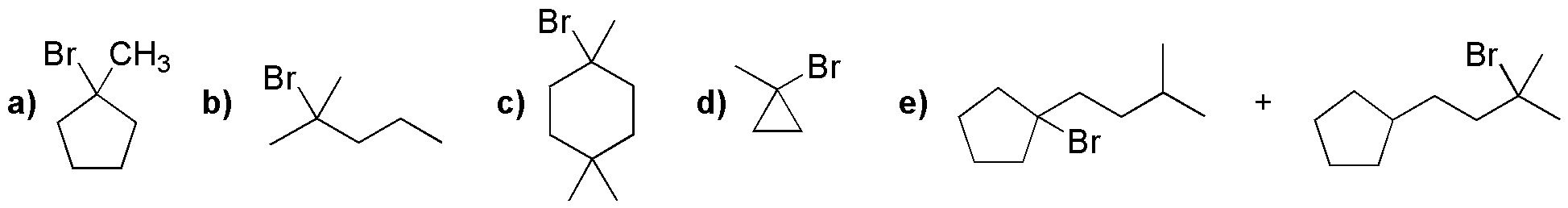
Solution 25:
The best choice would be compounds c and f, because they give in halogenation only one product, since all their hydrogens are equivalent.
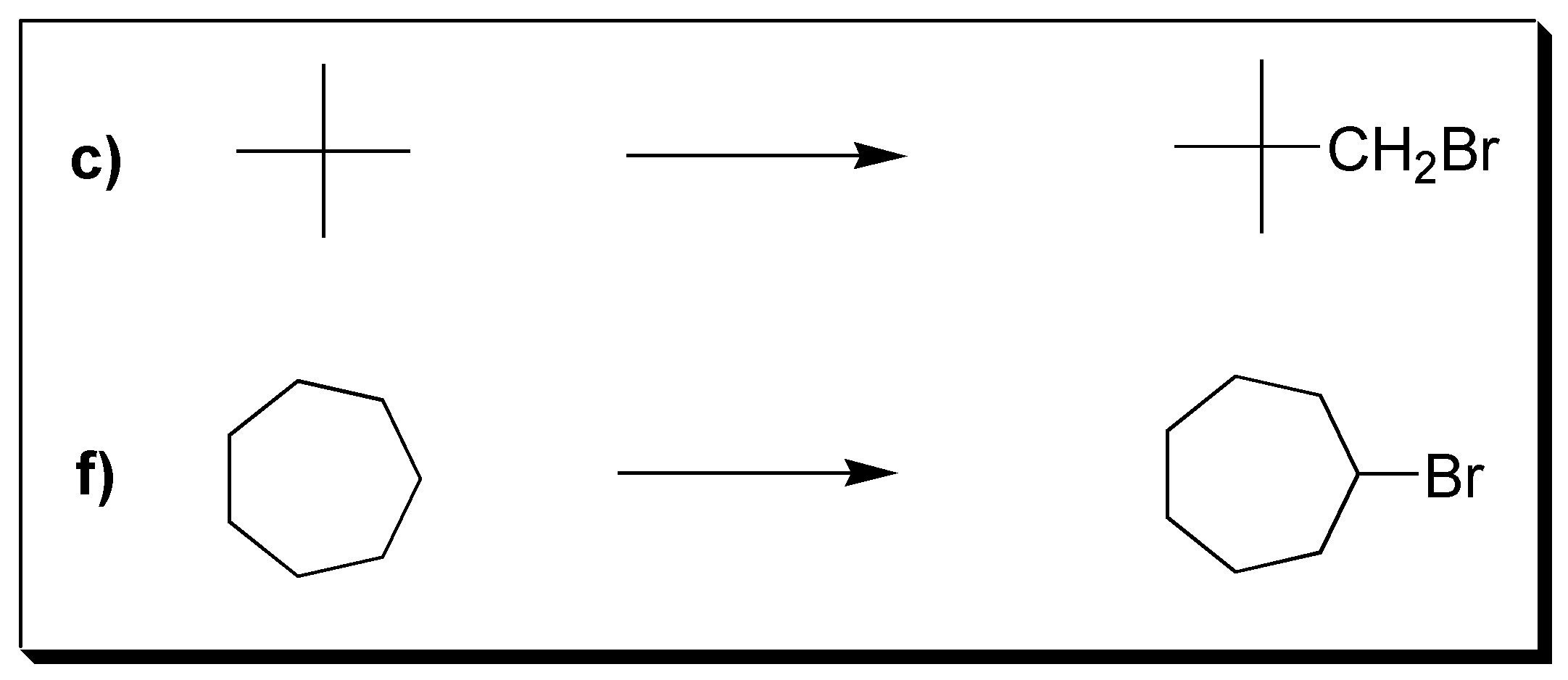
The rest give mixture of products, since they have different types of hydrogens (primary, secondary, tertiary).
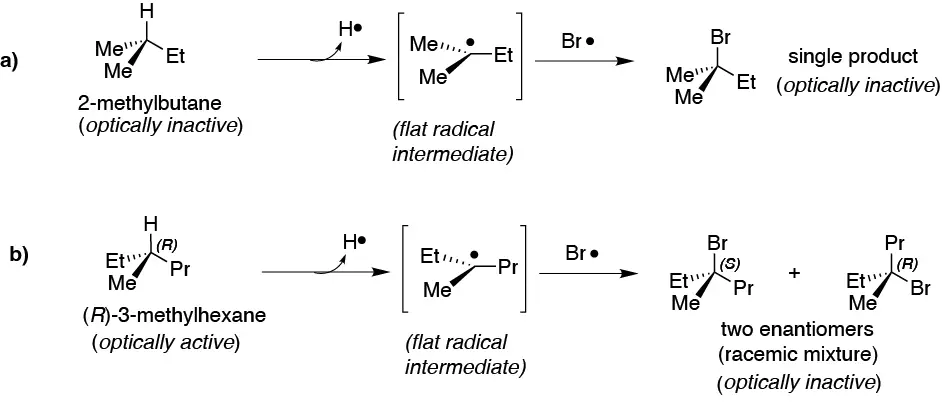
Solution 26:
The radical intermediate formed in both cases is planar and its radical bromination leads to the attack of the Br· radical by the two faces of the intermediate, leading to the formation of a single product in a) and of a racemic mixture in b). Both cases are optically inactive.
Solution 27:
(a) Fragmentation of propane gives the methyl and ethyl radicals. By reacting them, ethane, propane and butane can be obtained.
b) In the fragmentation of pentane the following radicals are obtained: methyl, ethyl, propyl and butyl. Recombination of these gives the products listed in the following table:
| methyl | ethyl | propyl | butyl | |
|---|---|---|---|---|
| methyl | ethane | |||
| ethyl | propane | butane | ||
| propyl | butane | pentane | hexane | |
| butyl | pentane | hexane | heptane | octane |
c) Pyrolysis of 2,3-dimethylpentane leads to the formation of: methyl, 1,2-dimethylpropyl and isopropyl. The recombination of these radicals is given in the following table:
| methyl | 1,2-dimethylpropyl | isopropyl | |
|---|---|---|---|
| methyl | ethane | ||
| 1,2-dimethylpropyl | 2,3-dimethylbutane | 2,3,4,5-tetramethylhexane | |
| isopropyl | 2,3,4-trimethylpentane | 2,2,3-trimethylbutane | 2,3-dimethylbutane |
Solution 28:
Taking into account the balance of matter between reactants and products one would have:
2C4H10 + 13O2 → 8CO2 + 10H2O
Solution 29:
Taking into account the balance of matter between reactants and products one would have:
2C12H26 + 25O2 → 24CO + 26H2O