Go to the page with the list of problems.
Alkenes and Dienes – solutions to problems
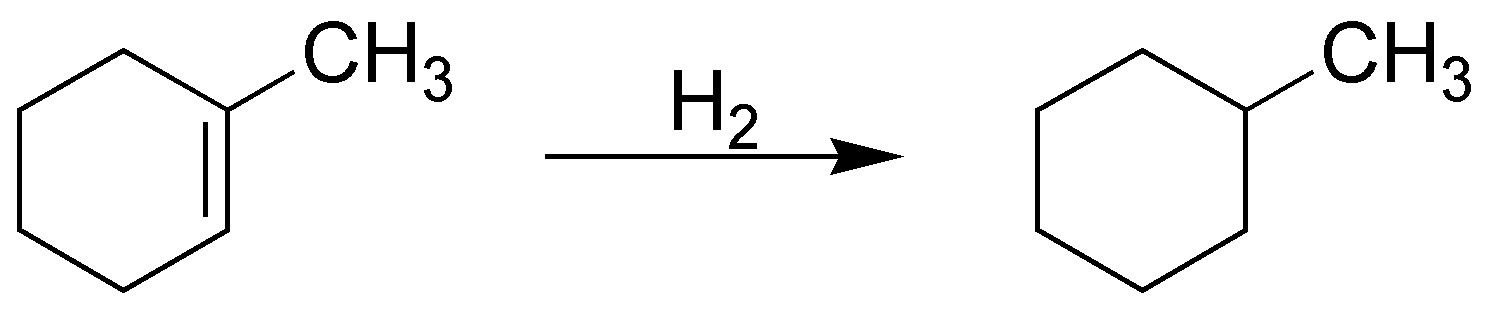
Solution 1:
The hydrogenation reaction, regardless of the stereochemistry of the reaction, will produce an alkane. In this case it is methylcyclohexane, which as we can see is a symmetrical and unique product (it has no stereoisomers).
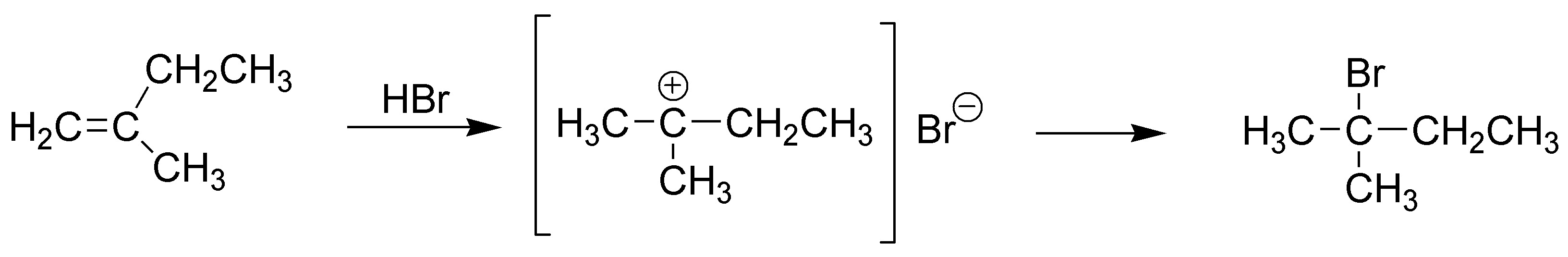
Solution 2:
The hydrohalogenation reaction starts by the attack of a proton from the acid on the π-cloud of the olefin to produce a carbocation (fulfills Markovnikov’s rule) and therefore the hydrogen will bond to the least substituted carbon, in this case carbon-1 producing a tertiary carbocation, which in turn reacts with the bromide ion giving 2-bromo-2-methylbutane.
Solution 3:
The halogenation reaction produces as an intermediate a cyclic bromonium ion, which is subsequently attacked by the bromide ion on the opposite side, producing in both cases a 2,3-dibromobutane.
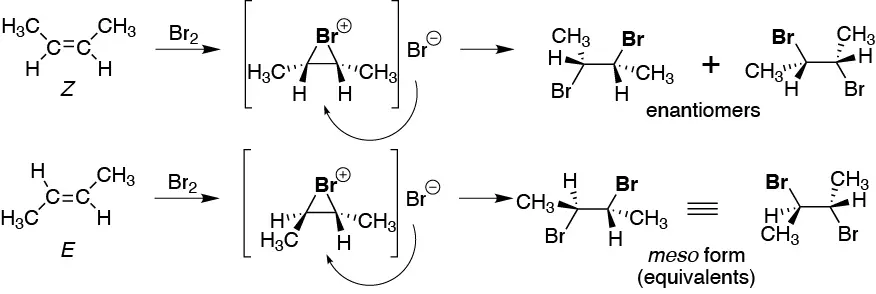
But whereas the Z (cis) isomer produces the enantiomer pair (2R,3R)/(2S,3S)-2,3-dibromobutane, the E (trans) isomer produces the (2R,3S)-2,3-dibromobutane which is a “meso” form and therefore is a unique product. When a reaction behaves in this way, i.e. the same reaction with two different stereoisomers yields different stereoisomers it is said to be stereospecific.
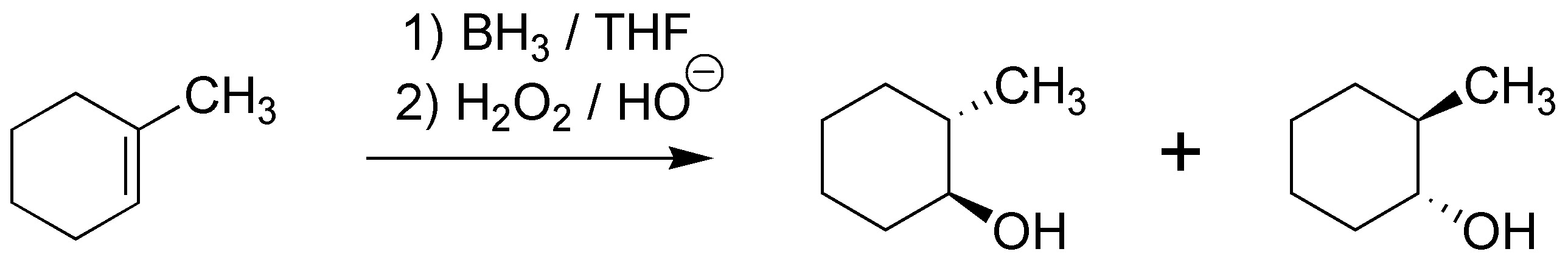
Solution 4:
The reaction of addition of borane in THF followed by hydrogen peroxide in basic medium produces an alcohol with the “anti-Markonvikov” regioselectivity and a “syn” stereoselectivity, i.e. the hydroxyl group will go to the least substituted carbon and the hydrogen to the most substituted and both groups must enter on the same face of the molecule thus yielding trans-2-methylcyclohexanol as a pair of enantiomers: (1R,2R) and (1S,2S).
Solution 5:
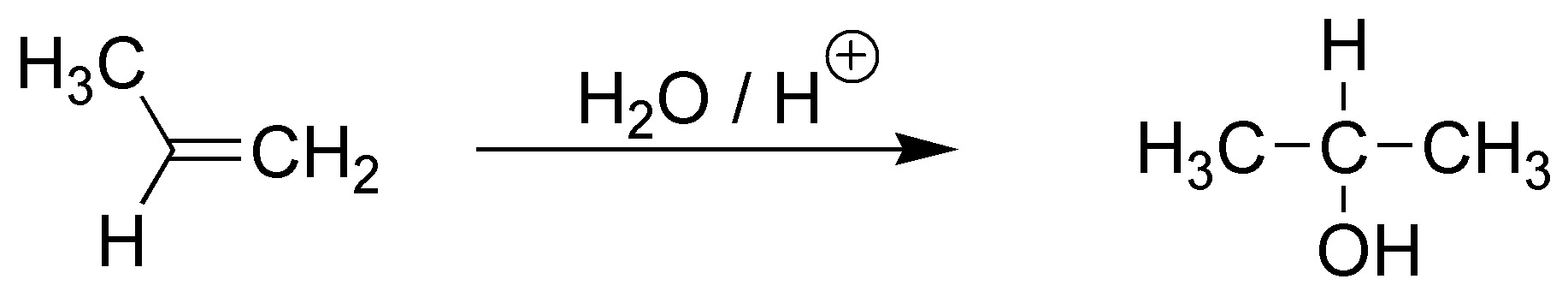
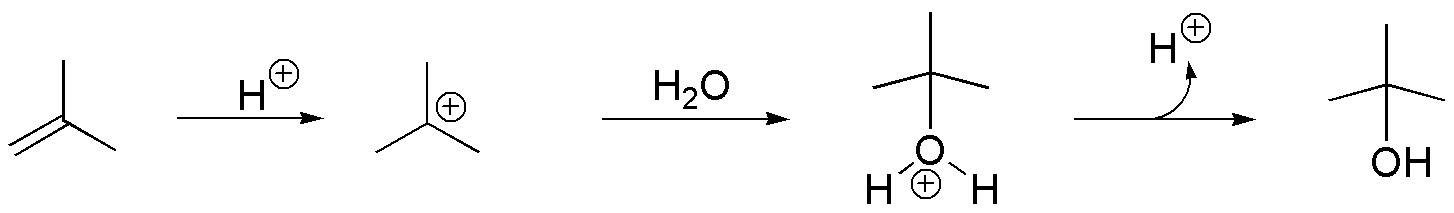
The acid-catalyzed hydration reaction starts by the attack of a proton from the acid on the π-cloud of the olefin to produce a carbocation.
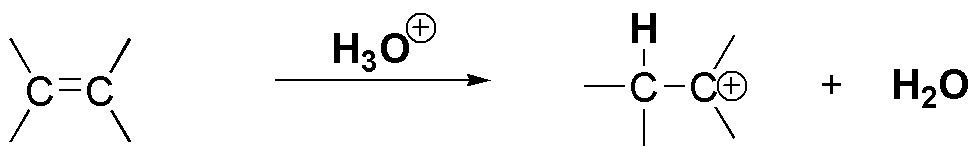
On the one hand, the electron density of the π-cloud increases, as the substitution of the double bond increases since the alkyl groups exhibit a slight electron donor effect. As the electron density increases, its reactivity towards electrophiles increases, but the most important reason is the type of intermediate generated in each case.
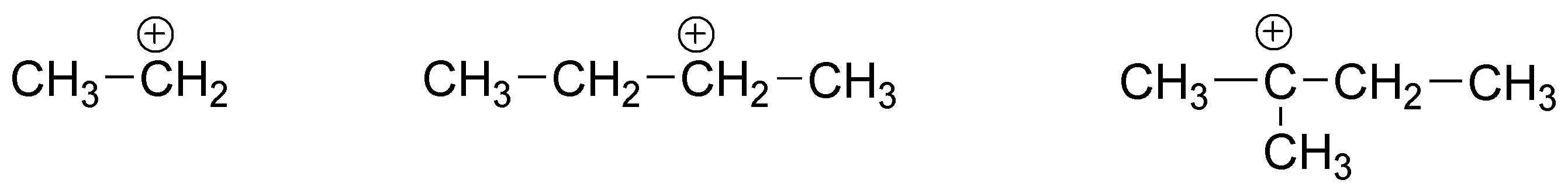
For ethene, a primary carbocation is produced, for (E)-but-2-ene a secondary carbocation and for 2,3-dimethylbut-2-ene a tertiary carbocation. As is known, the order of reactivity of carbocations is:
tertiary > secondary > primary > primary > methyl.
The different stability of these carbocations results in substantial changes in the activation energy, thus perfectly justifying the proposed reactivity order.
Solution 6:
A) Compound A is obtained by electrophilic reaction of HBr. A Markovnikov addition occurs giving 2-bromo-2-methylpropane (tert-butyl bromide).
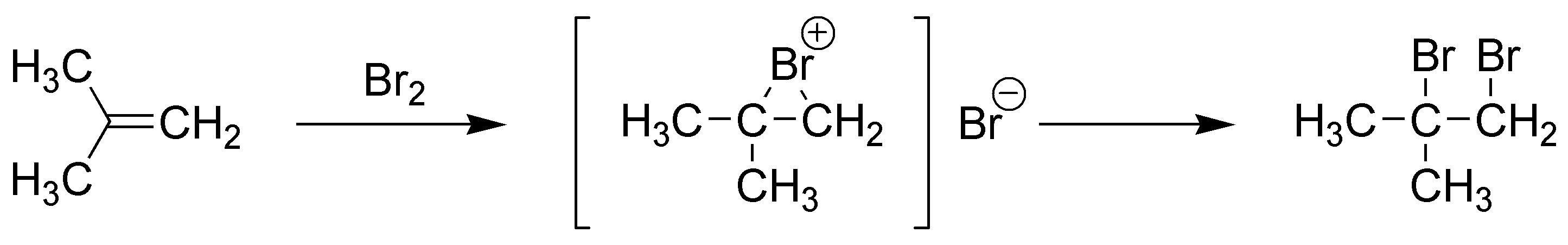
B) Reaction with Br2 leads to the formation of a dibromoalkane: 1,2-dibromo-2-methylpropane. The addition of bromine to alkenes is anti via an intermediate bromonium ion, the Br+ ion acting as an electrophile. Subsequently, the attack of the nucleophile in this case the Br– bromide ion takes place.
C) Permanganate is an oxidant that can give a glycol, in the case that the pH is neutral and the reaction is carried out at low temperatures, or produce the breakage of the double bond if the pH of the medium is basic and the reaction is carried out hot. In this case 1,2-dihydroxy-2-methylpropane would be produced.
D) The addition of bromine to alkenes is anti via an intermediate bromonium ion, the Br+ ion acting as an electrophile. Subsequently, water attack occurs as a nucleophile. The attack occurs on the most substituted carbon which has the highest positive charge density, in this case 1-bromo-2-methyl-2-propanol will be formed.
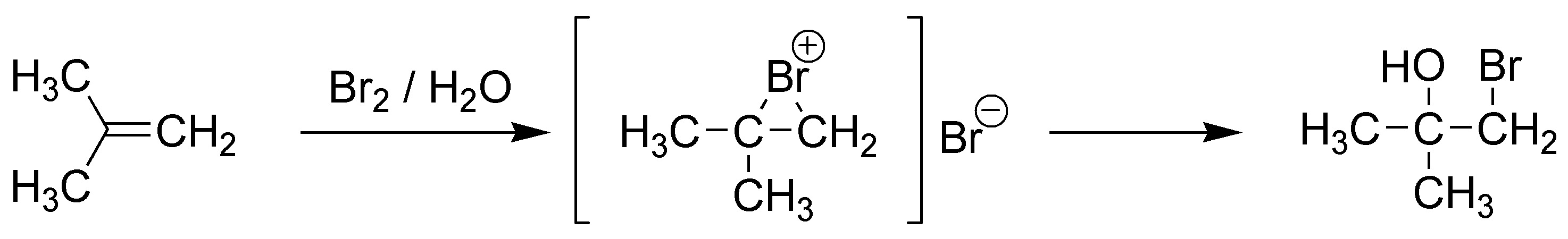
E) The reaction of methylsulfenyl chloride is an electrophilic Markovnikov-type reaction, in which the electrophile is methylsulfenyl (MeS+ and therefore will bind to the least substituted carbon. The chloride ion (nucleophile) will attack the most substituted carbon.
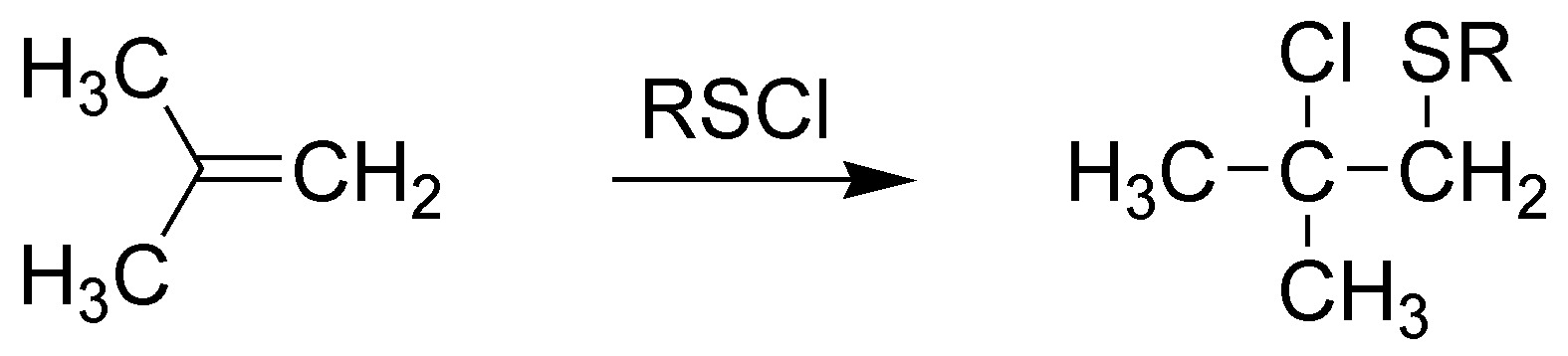
F) The reaction of an alkene with a peracid (in this case peracetic acid) leads to the formation of epoxides (oxacyclopropanes). In this case 1,1-dimethyloxycyclopropane would be formed.
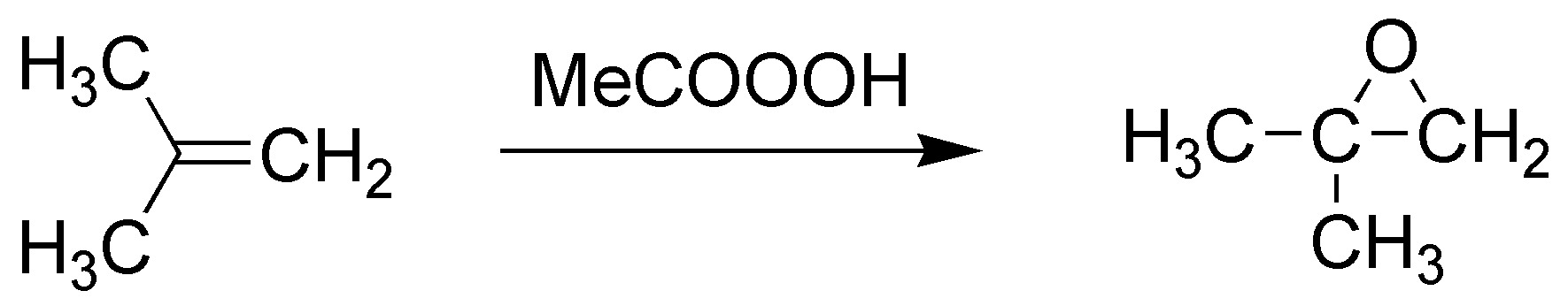
G) Ozonolysis of an alkene, followed by reduction leads to the breaking of the double bond producing two carbonyl compounds in this case formaldehyde and propanone (acetone).
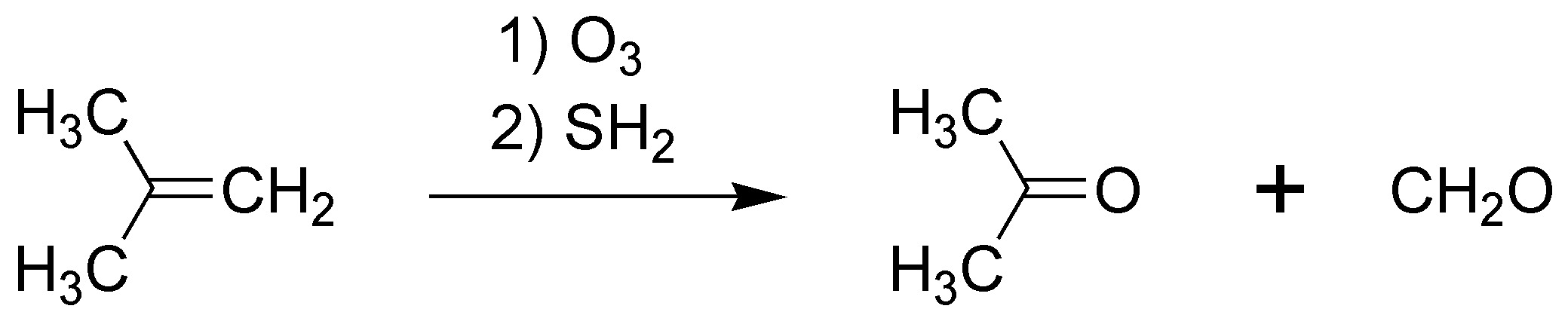
H) Treatment of an alkene with borane followed by oxidation in a basic medium produces as a final result the addition of anti-Markovnikov water to the alkene. The compound 2-methyl-propan-1-ol will therefore be formed.
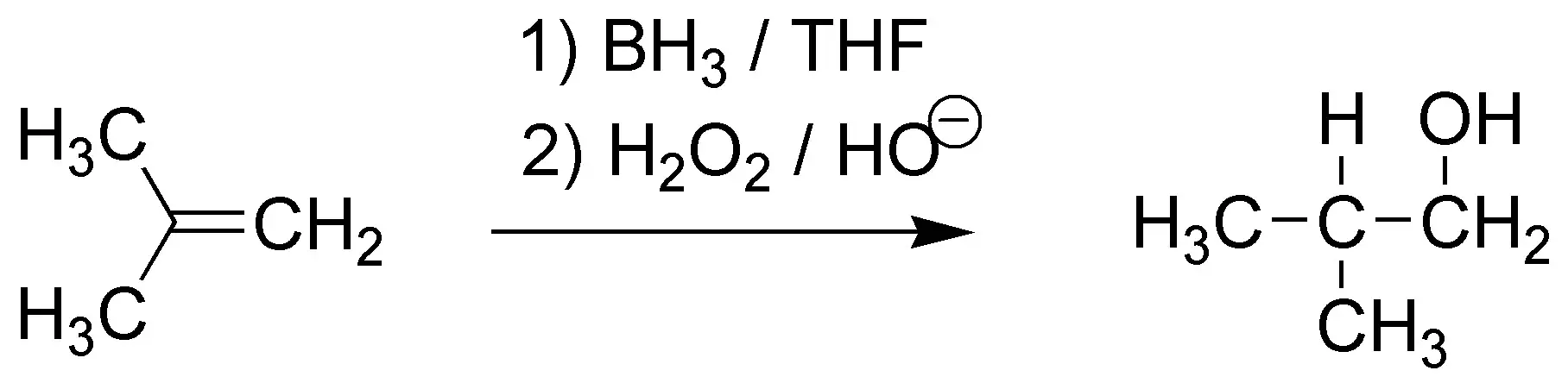
Solution 7:
As a general rule for this type of problems, we should always check the atomic balance between the initial and the final product. Subsequently, we must analyze if it is possible to perform the transformation in a single step or not and finally we must observe the selectivity of the transformation.
A) The atomic balance indicates that HBr has been added. We also observe that it has been added in such a way that the bromine is located on the least substituted carbon. It is therefore an anti-Markovnikov addition. Therefore, let us make the addition in the presence of peroxides and light, and the reaction will be carried out by a radical mechanism.
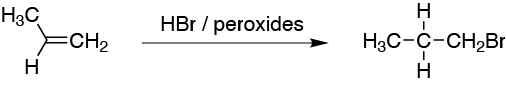
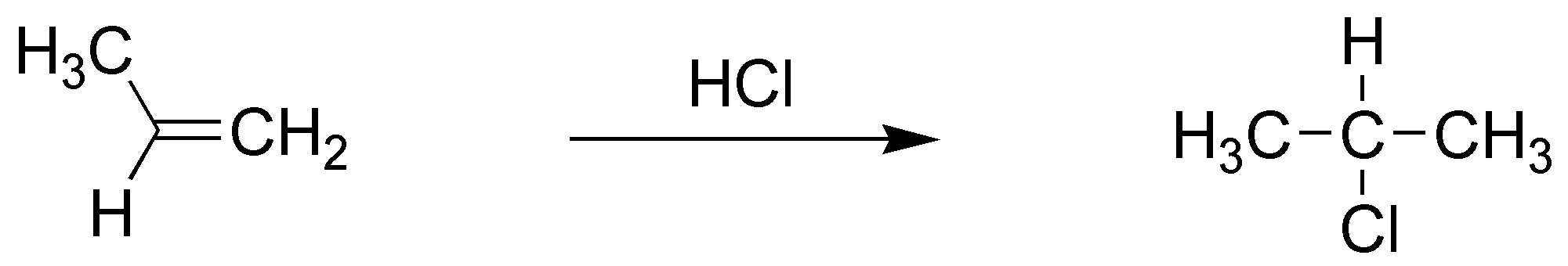
B) The atomic balance shows that HCl has been added. We also observe that it has been added in such a way that the chlorine is bonded to the most substituted carbon. It is therefore a Markovnikov type addition of HCl to an alkene.
C) The atomic balance indicates that two hydroxyl groups (2 OH) have been added. Therefore, the reagent will be either KMnO4 at pH=7 or OsO4 with sodium bisulfite (NaHSO3).
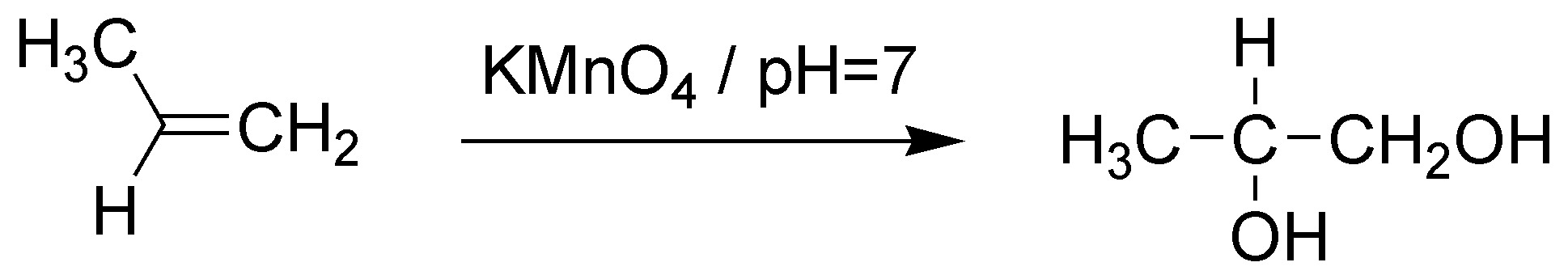
D) The atomic balance indicates that only one molecule of water has been added. Also, we observe that the hydroxyl is located on the most substituted carbon. It is therefore a Markovnikov type addition of H2O to an alkene, catalyzed by acid.
E) The atomic balance shows us that the initial molecule, with 4 carbon atoms, has been broken. Since the final product has only two carbons. As it also has 4 oxygen atoms, an oxidative breakage has occurred. We can make it with KMnO4 in acid medium.
F) The atomic balance suggests that the initial molecule has lost a methylene (CH2) and has gained an oxygen atom. It is therefore an oxidative breakage that we can perform by the corresponding ozonolysis and subsequent reduction of the ozonide formed.
Solution 8:
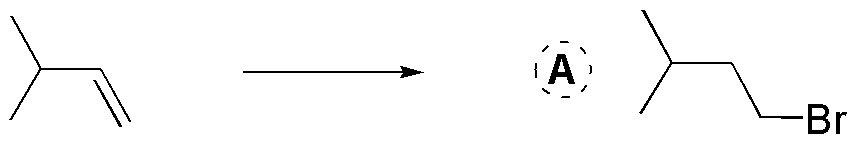
A) Compound A is obtained by reaction of HBr in the presence of a radical initiator such as benzoyl peroxide. An anti-Markovnikov addition occurs.
B) The reaction with HCl leads to the formation of an alkyl halide, via a Markovnikov addition. According to the proposed mechanism, the H+ acts as an electrophile, the most stable carbocation possible (the secondary) is produced and the chloride ion acts as a nucleophile, attacking the positively charged carbon to give the secondary alkyl chloride.
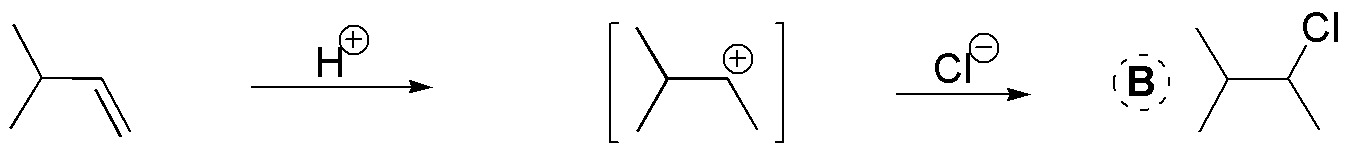
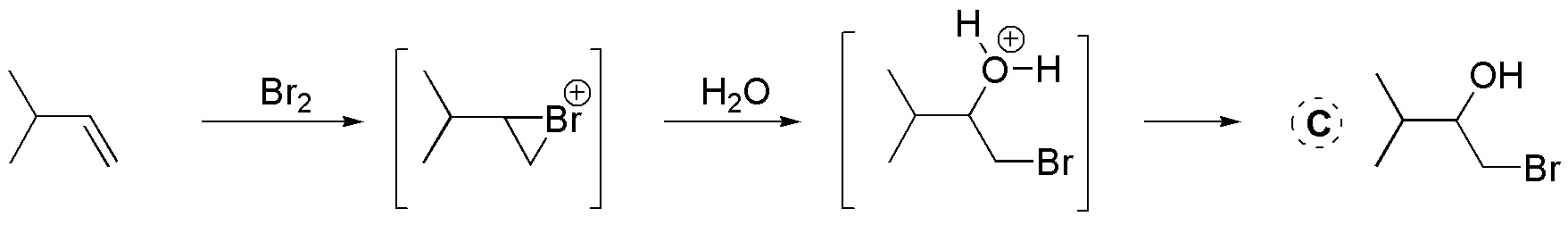
C) The addition of bromine to alkenes is anti through an intermediate bromonium ion, the Br+ ion acting as an electrophile. Subsequently, the attack of water as a nucleophile takes place. The attack occurs on the most substituted carbon, which has the highest positive charge density.
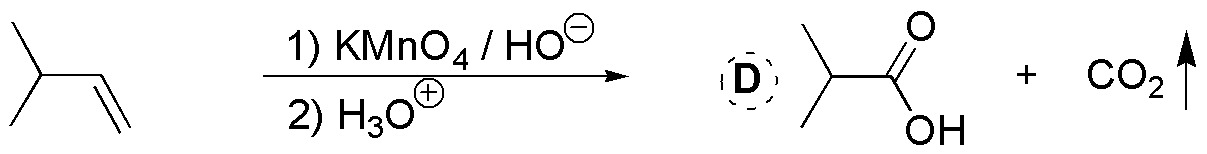
D) Permanganate is an oxidant that can give a glycol, if the pH is neutral and the reaction is carried out at low temperatures, or it can produce the breakage of the double bond if the pH of the medium is basic and the reaction is carried out hot, as in this case. Since in the starting alkene the unsaturation is at the end of the chain, two fragments are produced which are CO2 (gas) which is released in the reaction crude and is not detected, and the potassium salt of the corresponding carboxylic acid, since the oxidation is usually carried out in a basic medium. To isolate this carboxylic acid it is necessary to acidify the reaction crude, once the oxidation is finished.
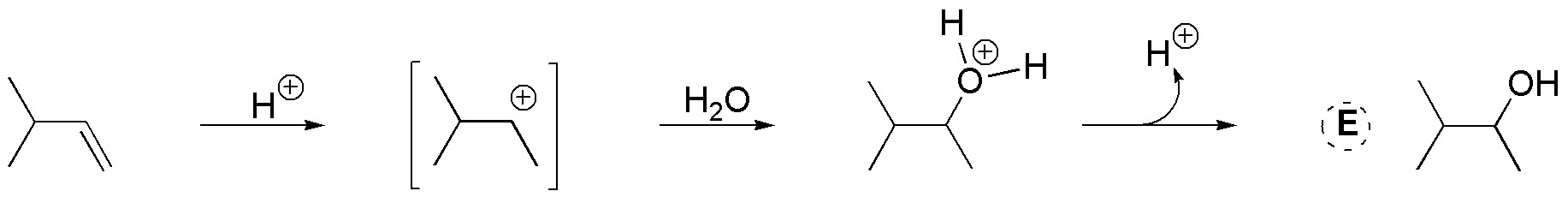
E) A proton of the sulfuric acid acts as an electrophile, generating a reaction intermediate which is a secondary carbocation. Since the sulfate ion has no nucleophilic character, water plays this role by attacking the carbon that has a formal positive charge. A secondary alcohol is obtained. (Note that there is no net consumption of protons, so the acid acts as a catalyst.)
F) Hydrogen has a reducing character and is added to double bonds stereospecifically by a syn addition (on the same side of the double bond) to give the corresponding saturated compound. In this particular case, since the double bond is located at the end of a carbon chain, the addition of hydrogen does not generate any new stereocenter.
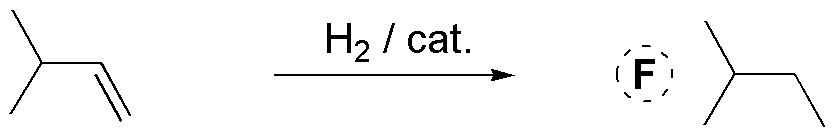
Solution 9:
Compound A. The addition of halogens to alkenes is an anti and therefore stereospecific process. Depending on the starting alkenes, stereocenters can be generated. Since the stereochemistry of the compound obtained is not specified in this case, the addition of chlorine leading to the formation of compound A can come from either II or III.
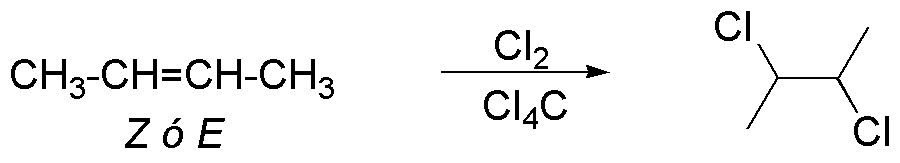
Compound B. It is the result of the addition of a water molecule to II or III by treatment of the corresponding alkene with an aqueous solution of an acid with a weakly nucleophilic anion (SO4=, PO43-). Regardless of whether the alkene is Z or E, the attack of the electrophile (proton) produces the same carbocation, which is attacked by a water molecule acting as a nucleophile.
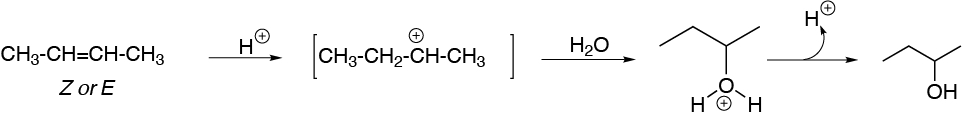
Compound C. A procedure similar to that described in the previous section applied to I leads to the formation of C
Compound D. The addition of anti-Markovnikov-type HBr on I allows the preparation of D. This requires the use of a radical initiator such as benzoyl peroxide or illumination with suitable light to generate the Br· radical necessary to initiate the reaction.
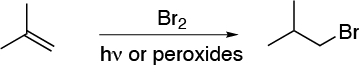
Compound E. Oxidative cleavage of alkenes with potassium permanganate (hot) generates two fragments. Depending on the type of substitution in the double bond, the nature of the fragment will vary. If the RHC= grouping is on one side of the double bond, the carboxylic acid R-CO2H is obtained. If the double bond is at the end of a chain (=CH2) CO2 is produced, and if one of the carbons of the double bond is attached to two alkyl groups (R1R2C=) a ketone (R1R2C=O) is obtained. The stereochemistry of the double bond (Z or E) does not influence the nature of the fragment.
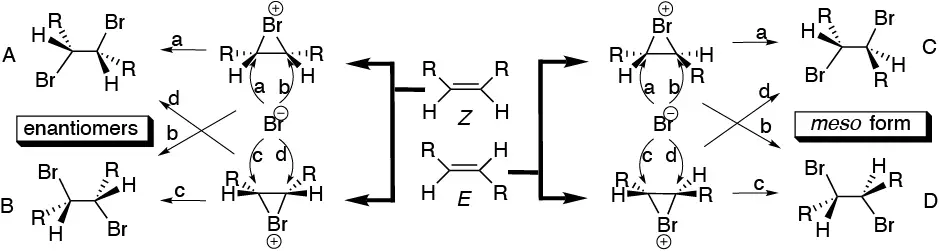
Compound F. With respect to stereochemistry, the addition of bromine to a double bond is one of the most complex processes within addition reactions.
In the following figure, the addition of bromine to a generic alkene of the type R-CH=CH-R of configuration Z or E is described. For compound Z an enantiomer pair is obtained, while for E a meso compound is obtained, when the two R groups attached to the double bond are equal.
All the possibilities of bromine addition to alkenes of type E or Z are summarized. In compound E the bromine atoms are placed in an antiperiplanar arrangement, which is how these atoms remain when a bromide ion attacks the intermediate bromonium ion of the reaction. From this situation it can be deduced that the arrangement of the methyl groups in the Newman projection is the starting one, that is to say, the double bond has a Z configuration. The structure depicted is one of the enantiomers obtained on the addition of bromine on the starting olefin III.
Solution 10:
a) The addition of hydrogen halides to alkenes always gives the Markovnikov addition product.
False: When peroxides are present, or it is illuminated, the mechanism is radicalic, obtaining the anti-Markovnikov product.
b) Ozonolysis of an alkene produces two carbonyl compounds.
True: Ozonolysis of alkenes produces aldehydes or ketones, even if the double bond is located at the end of a chain, formaldehyde is formed, in all cases carbonyl compounds.
c) The acid-catalyzed hydration of alkenes presents a stereochemistry without.
False: The reaction intermediate is a carbocation and therefore planar. The nucleophile (H2O), can attack indistinctly on both sides, so it is not correct to speak of syn or anti attack.
d) The addition of halogens to alkenes and the formation of halohydrins are anti additions.
True: In both cases a cyclic halonium intermediate is formed. The nucleophile, X–, for halogen addition and H2O for halohydrins attacks on the opposite side to that of the halogen, thus an anti addition.
Solution 11:
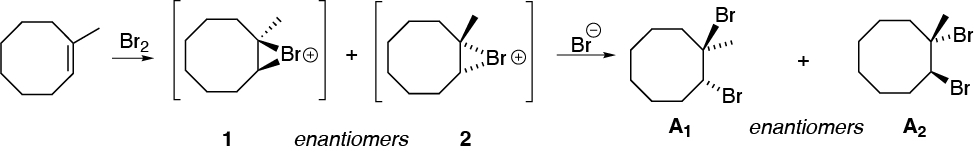
A) The addition of bromine onto 1-methylcyclooctene is stereospecific, as with all alkenes, since only two of the four possible stereoisomers are formed. The relative stereochemistry of the bromine atoms being added is trans, implying that the addition is anti.
The reaction begins with the formation of an intermediate bromonium ion as a result of the attack of the bromine molecule. There is no preference in the formation of the intermediate on either side of the double bond, so bromonium ions 1 and 2 are obtained in the figure. Next, the bromide ion is attacked on the opposite side where the bromonium ion is located and on the most substituted position, since this is where there is the highest density of positive charge. The final product of the reaction is actually an equimolecular mixture of the enantiomers A1 and A2, i.e. a racemic.
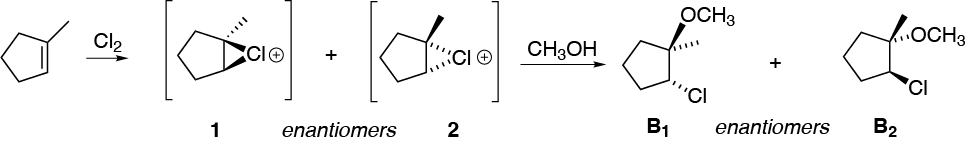
B) The reaction of 1-methylcyclopentene with chlorine in the presence of methanol generates, in the first instance, a cyclic chloronium ion, similar to the previous case. The intermediate chloronium ion is attacked mainly by the nucleophile that is present in greater proportion (methanol acting as solvent).
The reaction is regioselective, since methanol binds to the most substituted carbon, since it has a higher electron density due to the inductive effect of the methyl group. As a result, B is obtained as a mixture of enantiomers (B1 + B2).
C) When 2,4-dimethylpent-2-ene reacts with borane, an alkylborane is produced by syn addition placing the boron on the least substituted carbon. From the treatment with hydrogen peroxide in basic medium an alcohol is obtained, the overall process being an anti-Markovnikov addition.
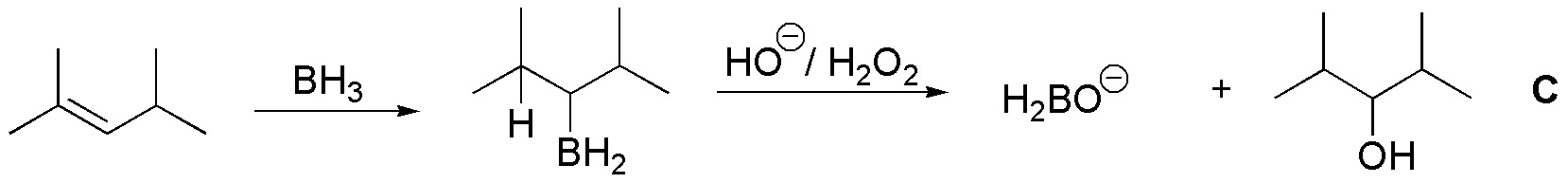
Since borane can transfer three hydrogens, one mole of borane can transform 3 moles of alkene, obtaining as a by-product the corresponding salt of boric acid.
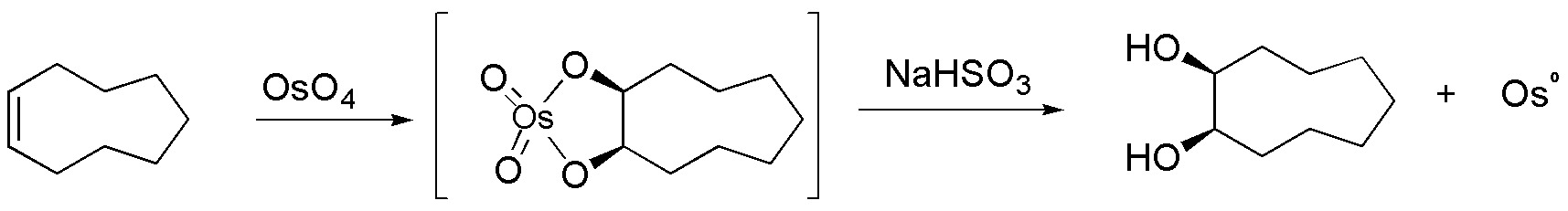
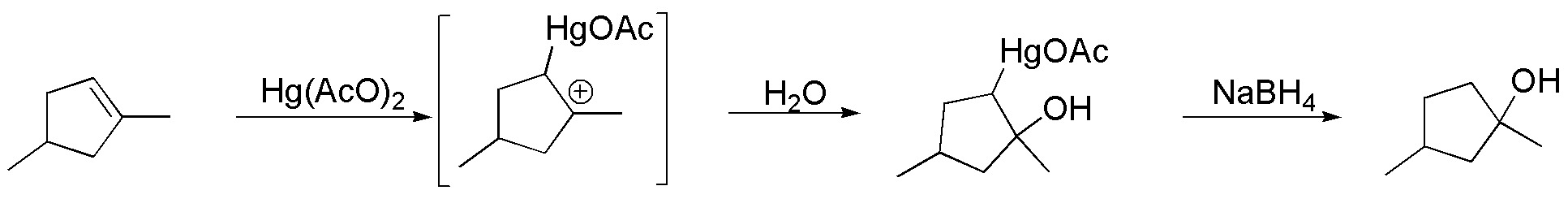
D) Cyclononene reacts with OsO4 by hydroxylation without. A metalloacyl intermediate is formed which is hydrolyzed with sodium bisulfite giving Osº.
E) The reagents used in this reaction are a non-nucleophilic mineral acid such as H3PO4 and KI. The mineral acid provides protons which when added to the alkene form the more stable carbocation. The I– ion has nucleophilic character and produces alkyl iodide.
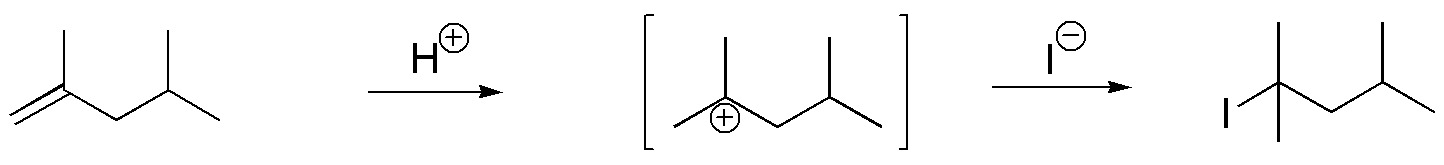
F) The reaction conditions described correspond to the mercuration-desmercuration reaction. The reaction is an addition leading to the formation of the Markovnikov product, through the formation of a so-called mercurial, which by reaction with NaBH4 leads to the formation of an alcohol, avoiding possible transpositions.
Solution 12:
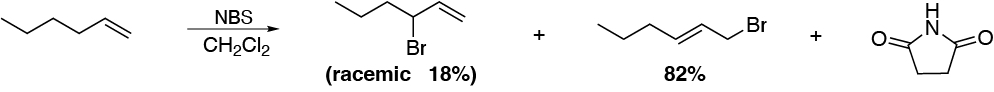
The reaction described is an allylic halogenation using N-bromosuccinimide as the reagent, which releases Br· radicals by homolytic cleavage of the N-Br bond. In principle, one would expect the major product to be 3-bromohex-1-ene, however, 1-bromohex-2-ene is obtained with 82 %. The justification is to be found in the reaction intermediate. A radical is formed which is actually a hybrid by resonance between two boundary forms and is therefore relatively stable.
![]()
The Br· radical can attack carbons 1 or 3. The major product comes from the attack at position 1 of the carbon chain.
Solution 13:
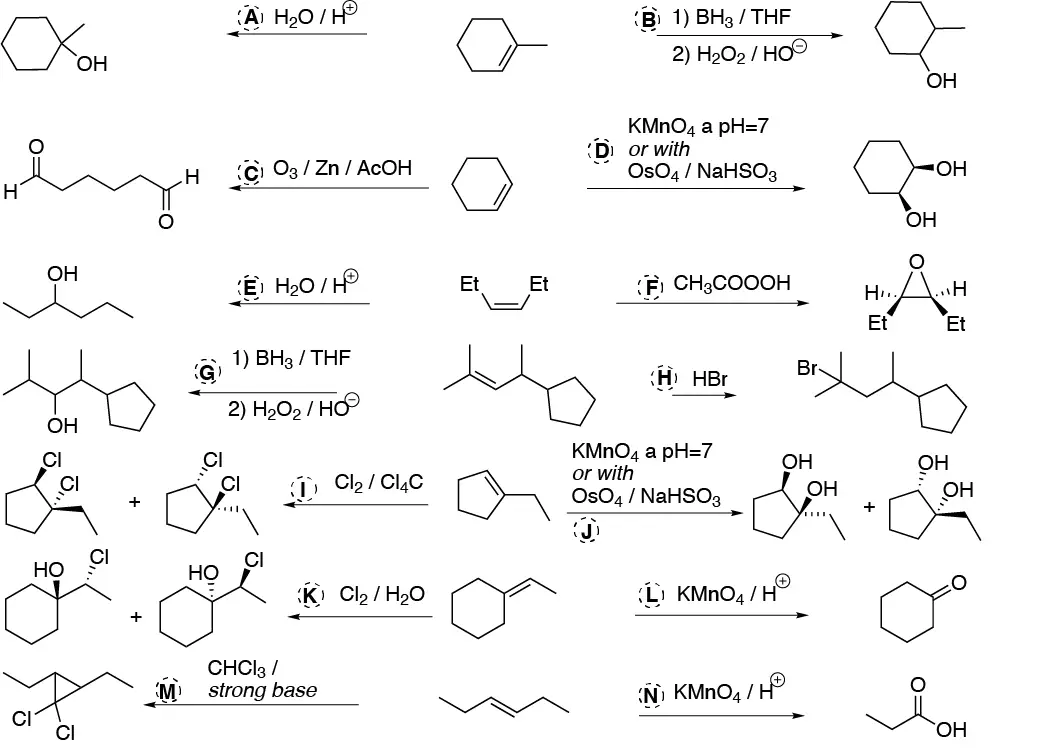
- A and B) The addition of water on 1-methylcyclohexane can be done in a Markovnikov (as in A) or anti-Markovnikov mode(as in B) so the conditions to perform such transformations will be: for A H2O / H+ and for B) hydroboration (BH3 / THF) and subsequent oxidation (H2O2 / OH–).
- C) The oxidative cleavage of cyclohexene to a dialdehyde involves ozonolysis and subsequent reduction of the ozonide with a reductant such as Zn/AcOH.
- D) Conversion to a diol (dihydroxylation) can be performed by KMnO4 at pH=7 or with OsO4 / NaHSO3.
- E) Conversion of 3-(Z)-hexene to 3-hexanol involves hydration in acid medium of the same.
- F) Conversion to an epoxide (oxacyclopropane) involves oxidation with a peracid, e.g. peroxyacetic acid (CH3COOOH).
- G) Involves hydration of the anti-Markovnikov type alkene so we must use hydroboration/oxidation as in B.
- H) Conversion involves Markovnikov-type hydrobromination so we would use HBr in the absence of radical initiators.
- I) Chlorination (Cl2 / Cl4C) of the double bond has occurred.This is an anti addition and the final product will appear as a racemic mixture of the two possible enantiomers.
- J) Dihydroxylation can be performed as in D: with permanganate at neutral pH or by osmium tetroxide / sodium bisulfite. It is produced on both sides of the double bond
- K) ClOH has been added. It is a chlorohydrin formation reaction so it is performed with Cl2 in water, giving a mixture of stereoisomers.
- L) It is an oxidative degradation to ketone can be performed with ozone and reductant (Zn / AcOH) or with permanganate in acid medium.
- M) A cyclopropane has been produced so it is the addition of a carbene. It is carried out with chloroform in strongly basic medium.
- N) It is an oxidative degradation to carboxylic acid so it will be carried out with KMnO4 in acid medium.
Solution 14:
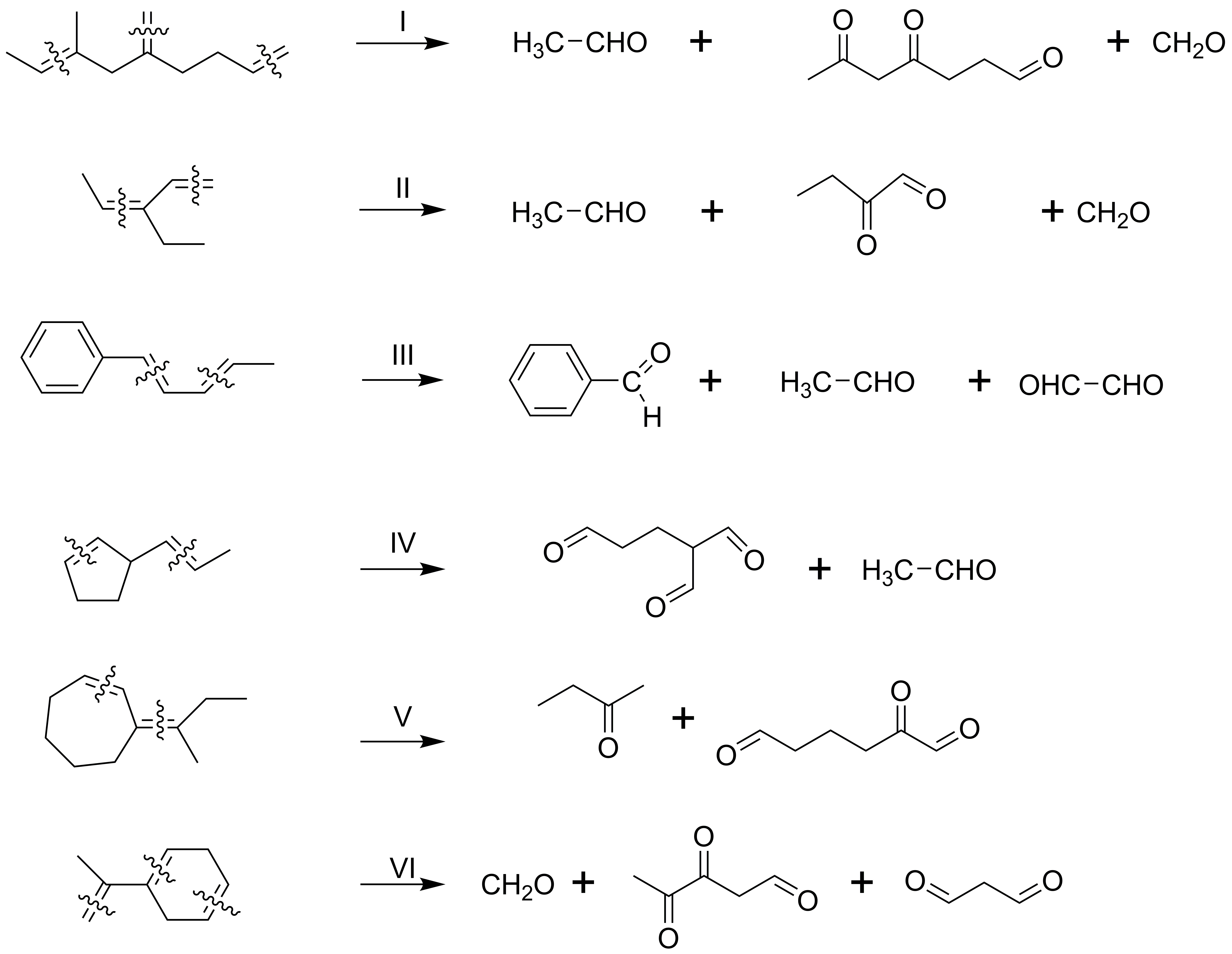
- I) Ozonolysis involves oxidative cleavage of all the double bonds of the molecule producing the corresponding carbonyl compounds would be formed, thus formaldehyde (2 moles), acetaldehyde and 4,6-dioxaheptanal.
- II) It would give formaldehyde, acetaldehyde and 2-oxabutanal.
- III) It would give benzaldehyde, glyoxal (CHO-CHO) and acetaldehyde.
- IV) It would give acetaldehyde and trialdehyde.
- V) It would give butanone and 2-oxa-heptanedial.
- VI) It would give Formaldehyde and methylphenylketone (acetophenone).
Solution 15:
In all cases the reaction begins by the attack of an electrophile (E+) on the conjugated double bond system. This attack of the electrophile occurs at one end of the conjugated diene.

The intermediate is a resonance hybrid, in which there is a higher density of positive charge on positions 2 and 4 of the conjugated system, and it is precisely there where the attack of the nucleophile occurs. In view of the reagents used the result of the reaction for each case will be as follows:
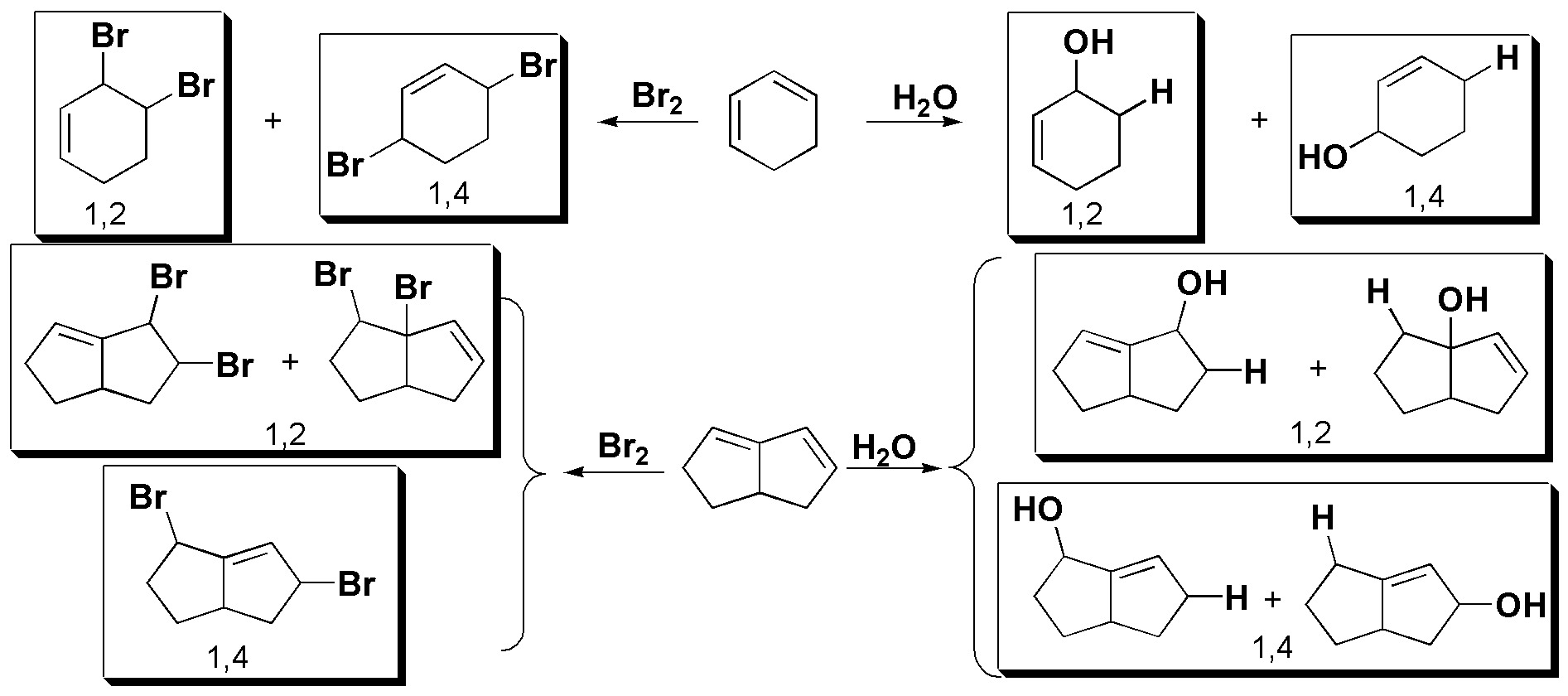
(Note that in the case of symmetrical dienes the reaction is simpler, whereas in asymmetrical dienes mixtures of products can occur).
Solution 16:
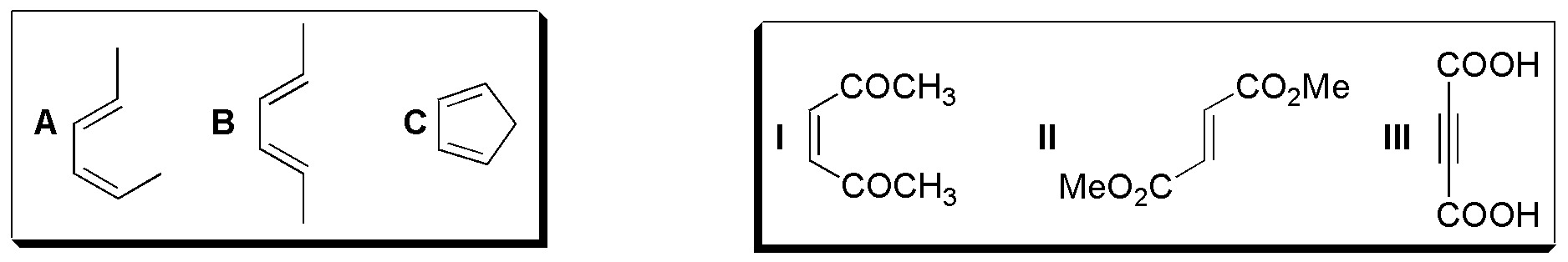
In solving this exercise the following considerations must be taken into account, since the reaction is stereospecific for both the diene and the dienophile:
- If the substituents on the double bonds of the diene are E and Z as occurs in diene A, they appear on opposite faces of the adduct.
- If the substituents on the double bonds of the diene are both Z, as is the case with B, they are located on the same face of the adduct.
- The cis-substituents on the dienophile, as with I, appear on the cis-configuration adduct.
- The trans substituents of the dienophile, as with II, appear on the trans-configuration adduct.
- In cyclic dienes the endo-type attack is more favored than the exo.
- When chiral products are formed, pairs of enantiomers are obtained since under conventional reaction conditions there is no asymmetric induction.
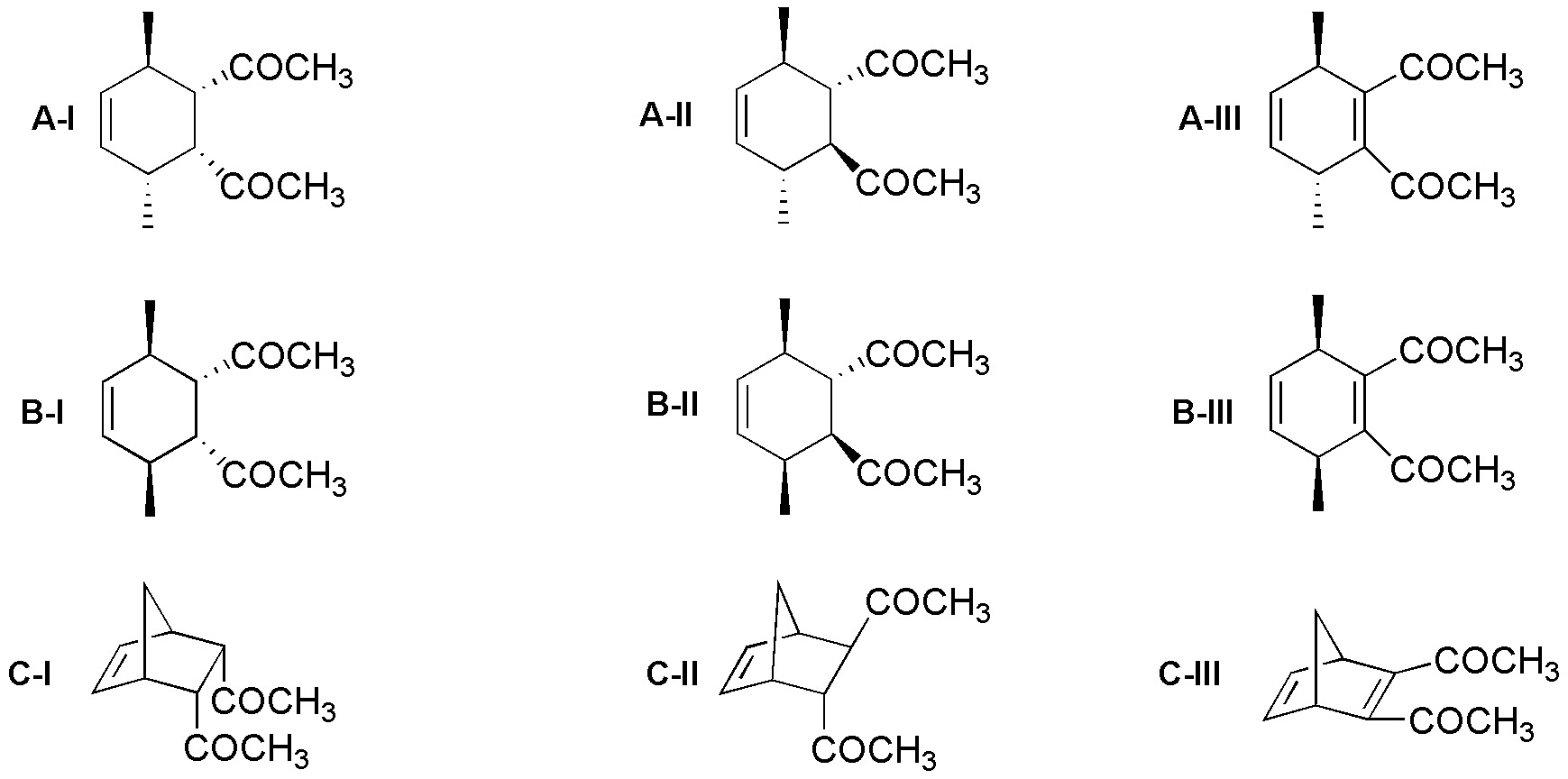
Compounds A-I, A-II, A-III, B-II and C-II are obtained as an equimolecular mixture of the depicted structure and its mirror image, i.e. as a racemic.
Solution 17:
a) The preparation of a primary haloalkane from an alkene suggests the use of an anti-Markovnikov HX addition reaction on a double bond of the =CH2 (terminal) type. The only alkene that fulfills this condition is I.
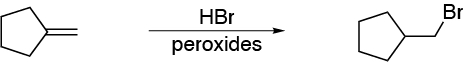
b) To obtain a secondary haloalkane, HX can be used as a reagent in a Markovnikov-type addition reaction (absence of peroxides). If I is used, the result is a tertiary haloalkane, for III, since there are two double bonds, the addition of one mole of HX leads to the formation of a haloalkene (one double bond remains) and if two moles are used, a mixture of dihaloalkanes is obtained which are constitutional isomers. The reaction described with II produces halocyclopentane which is obviously a secondary haloalkane.
![]()
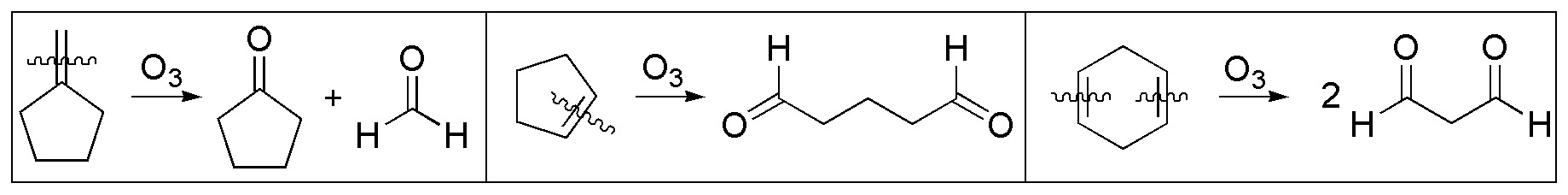
c) and d) Ozonolysis of alkenes leads to the formation of carbonyl compounds. The figure shows the products obtained by ozonolysis of the starting alkenes (I-III). To obtain a cyclic ketone we start from I and for a dialdehyde from II and III.
e) As mentioned in paragraph b), the addition of 1 mole of HX to III leads to the formation of a haloalkene.
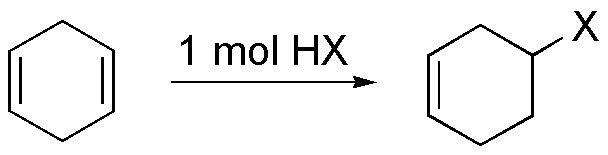
f) To obtain an alkane from an alkene, it is sufficient to choose the structure with the same carbon skeleton and by catalytic hydrogenation the necessary hydrogen is added on the double bond to generate the saturated compound, therefore, starting from II, cyclopentane is obtained.
![]()
g) The treatment of an alkene with potassium permanganate in a basic and hot medium produces what is called oxidative cleavage. If the double bond is of the -CH=CH- type, two carboxylic acids are obtained in the form of a potassium salt, since the pH is basic. To release the carboxylic acid, the reaction crude has to be acidified. If the double bond is of the -CH=CH2 type, a carboxylic acid, R-COOH and CO2 are generated. If the alkene is RR’C=CR”R””, two ketones are obtained (RR’C=O and O=CR”R””). The propanedioic acid is the result of the oxidative cleavage of one of the starting alkenes. The three breakage possibilities are indicated in the scheme, being only compatible with the statement if we start from III.
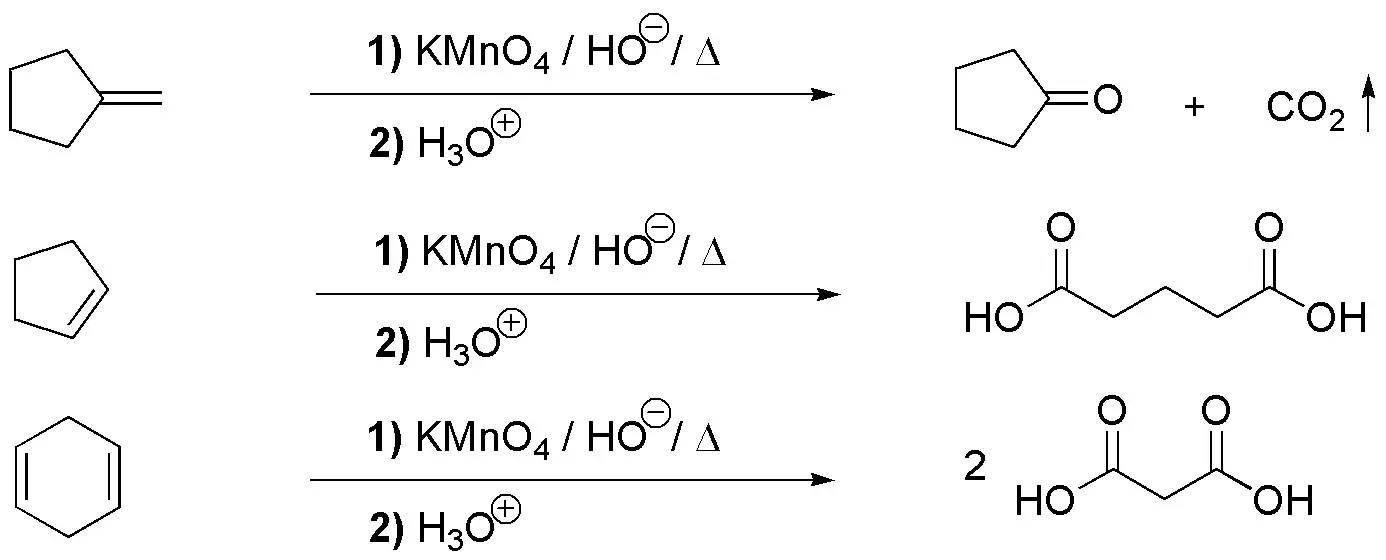
Solution 18:
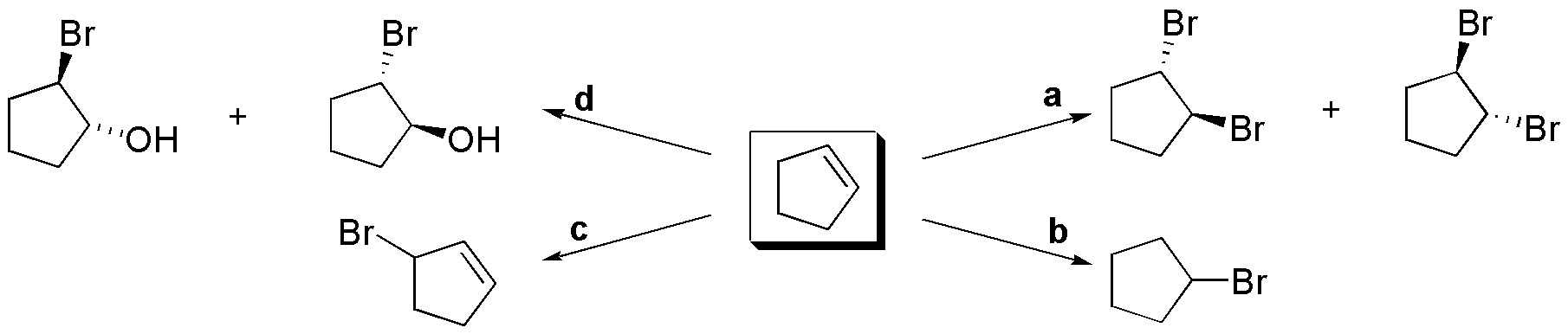
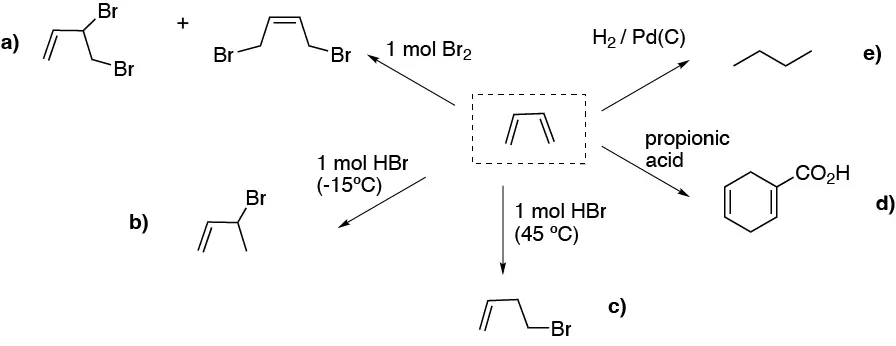
- a) Br2 in a solvent such as CH2Cl2 or CCl4 is necessary for this transformation. Under these conditions the anti addition of Br occurs, yielding a pair of enantiomers.
- b) The product is the result of the addition of HBr to the double bond. Addition in the presence of radical initiators or in the absence of radical initiators is indifferent in this particular case, since both Markovnikov and anti-Markovnikov addition produce the same compound.
- c) Treatment of cyclopentene with NBS in CH2Cl2, illuminating with suitable light to break the N-Br bond and produce the necessary radicals, allows the substitution of a hydrogen at the allylic position.
- d) To obtain this product it is necessary to treat cyclopentene with Br2 in aqueous medium. The intermediate bromonium ion is opened by the attack of water, giving a pair of enantiomers.
Solution 19:
From 2-methylpropene a tertiary carbocation is produced which acts as an electrophile on a second alkene molecule, giving a second carbocation, which again attacks a third 2-methylpropene molecule. The reaction ends with the loss of a proton to give a double bond.

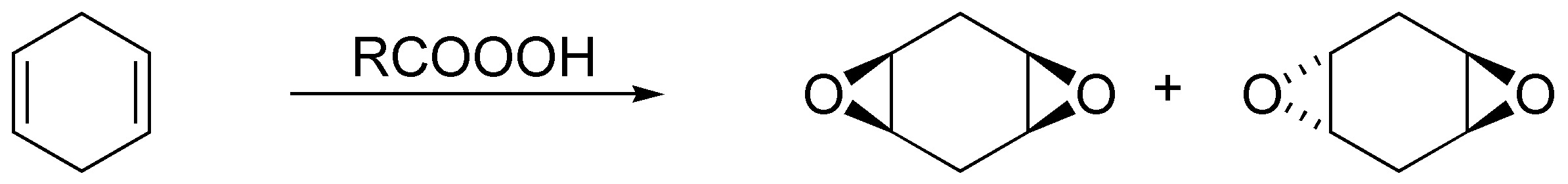
Solution 20:
The larger the substitution on a double bond the greater, is the electron density due to the inductive effect of the alkyl radicals. That results in an increase in the rate of oxygen attack of the peroxyacid in the epoxidation reaction.
Solution 21:
| Reagent | Reaction | Properties |
| HBr / peroxides | radical addition | Br· and H· radicals |
| H2S | ozonolysis | reductant |
| B2H6 | electrophilic addition | reductant; transfers H |
| RCO3H | epoxidation | oxidant |
| O3 | ozonolysis | oxidant |
| HCl | electrophilic addition | H+ (electrophile); Cl– (nucleophile) |
| H3PO4 | hydration of alkenes | H+ (electrophile) |
| H2O | hydration of alkenes; formation of halohydrins | nucleophile |
| H2 | catalytic hydrogenation | reductant |
| CH2I2 | cyclopropanation | generates carbenes |
Solution 22:
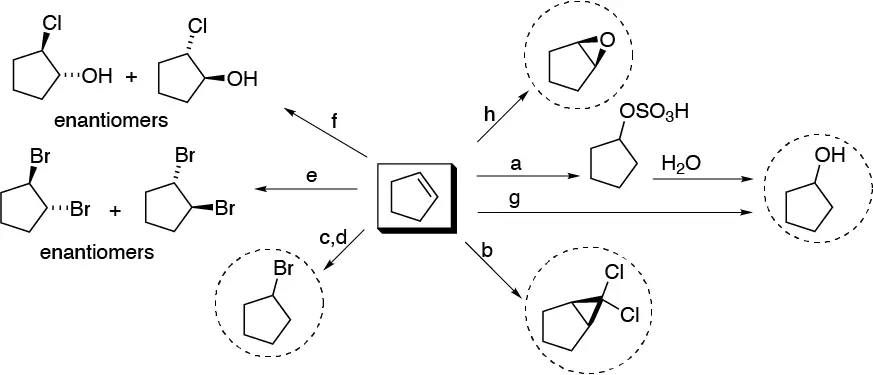
The molecules highlighted in the figure with dashed circles are achiral.
Solution 23:
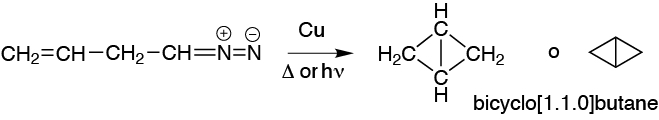
When irradiated, heated or put in the presence of copper a diazocompound generates a carbene which is added to the double bonds generating a cyclopropane:
Solution 24:
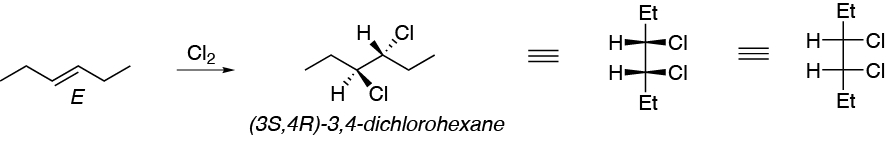
This is an anti addition and therefore:
Solution 25:
Hydration reaction b) and hydrogenation reaction d) would give the product shown from either of the two starting alkenes Z or E (I or II). The first reaction is a halogenation and only compound (I) can be used as starting reagent. To obtain product C by epoxidation (c) we need the starting alkene (II).
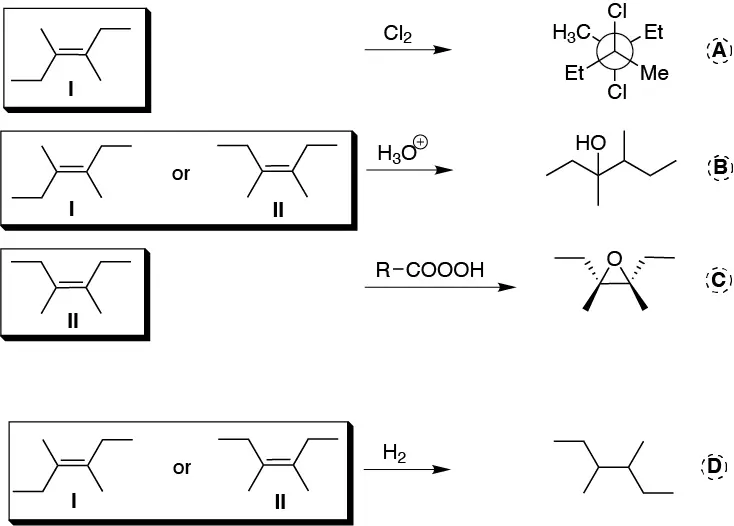
Solution 26:
- a) Markovnikov addition: it can be related to reactions I) and II), mercuration / demercuration and hydration of alcohols, since the overall result of it is the introduction of the hydroxyl group on the most substituted carbon (less hydrogenated).
- b) stereospecific process: catalytic hydrogenation (III) is stereospecific as it is a syn addition.
- c) anti addition: the process I) mercuriation / demercuriation, is so because the OH and the mercury atom enter from opposite sides of the alkene.
- d) anti-Markovnikov addition: The process IV) hydroboration / oxidation has an anti-Markovnikov regioselectivity because the overall result is the introduction of the hydroxyl group on the less substituted carbon (more hydrogenated).
Solution 27:
This will be an anti stereochemistry addition so in both cases the indicated product will be obtained as a pair of enantiomers:
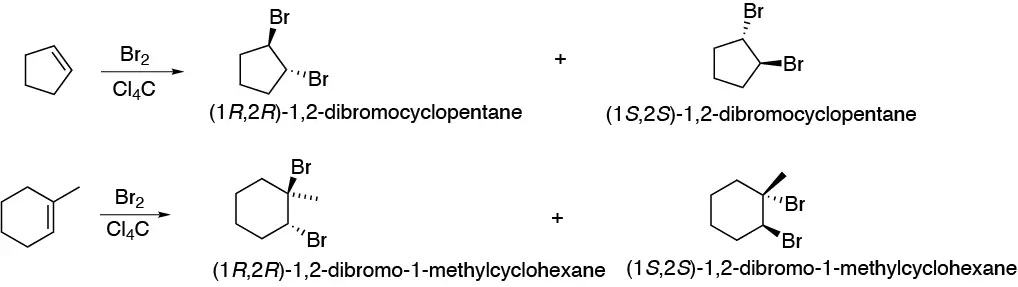
Solution 28:
The addition of halogens in aqueous solution leads to the corresponding halohydrins, which will appear as a mixture of the 8 possible stereoisomers (2 pairs of enantiomers for each isomer) taking into account that there is no regioselectivity in the reaction:
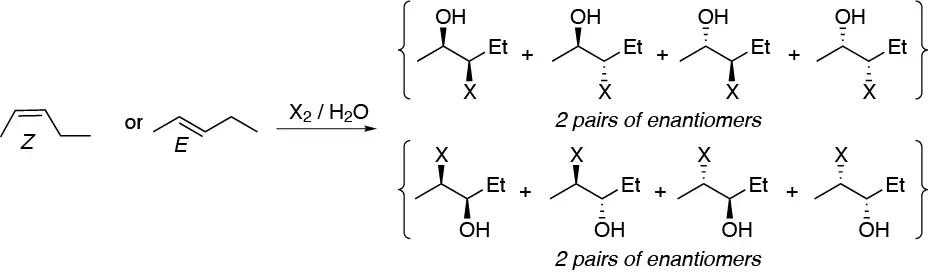
Solution 29:
Diazomethane when reacted with alkenes produces cyclopropanes in a stereospecific manner. In the case of cycloheptene as a single product by possessing a plane of symmetry and in the case of methylcyclohexene and (E)-but-2-ene as a pair of enantiomers, by not possessing one:
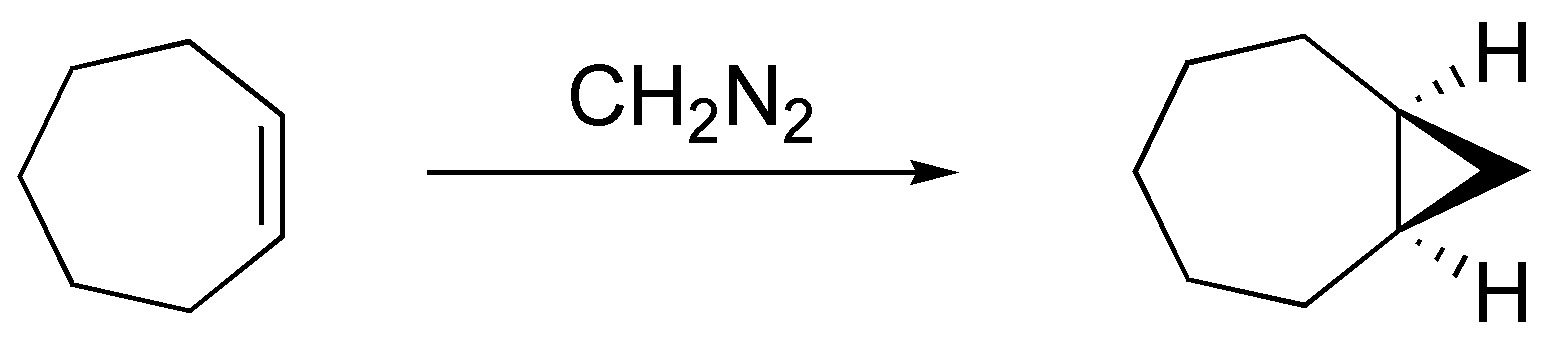
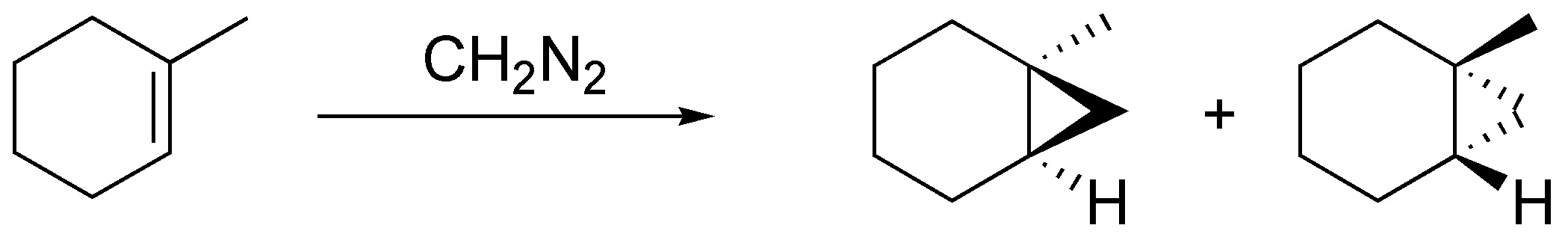
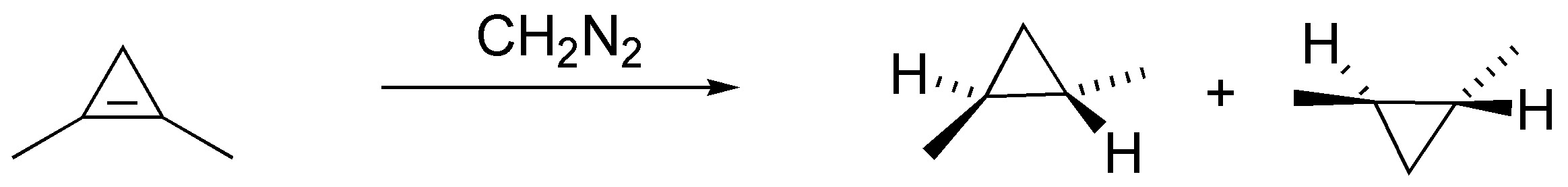
Solution 30:
The peracids generate with the alkenes epoxides (oxacyclopropanes) being a stereospecific reaction:
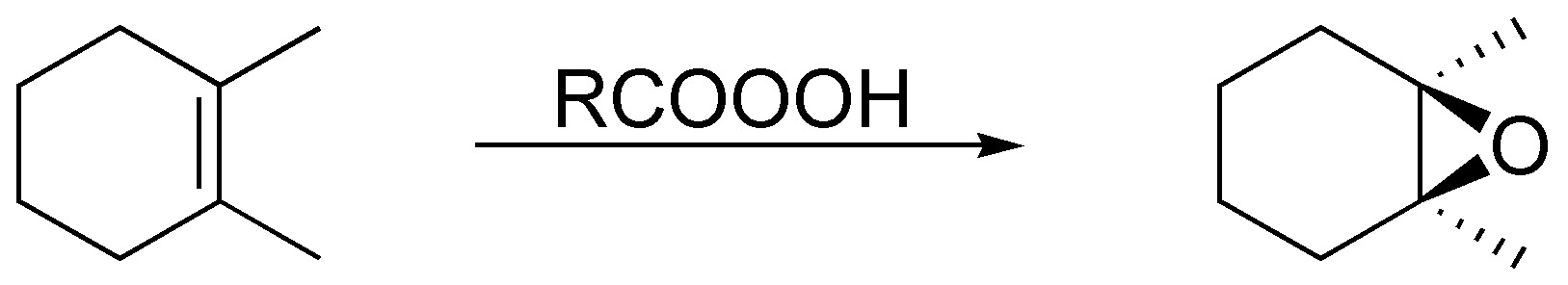
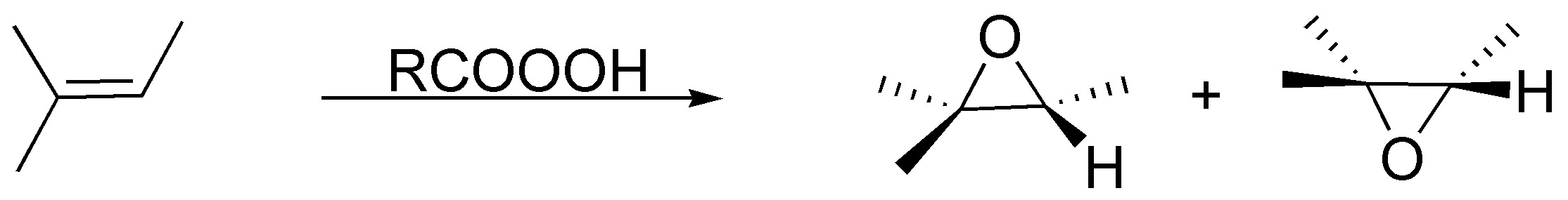
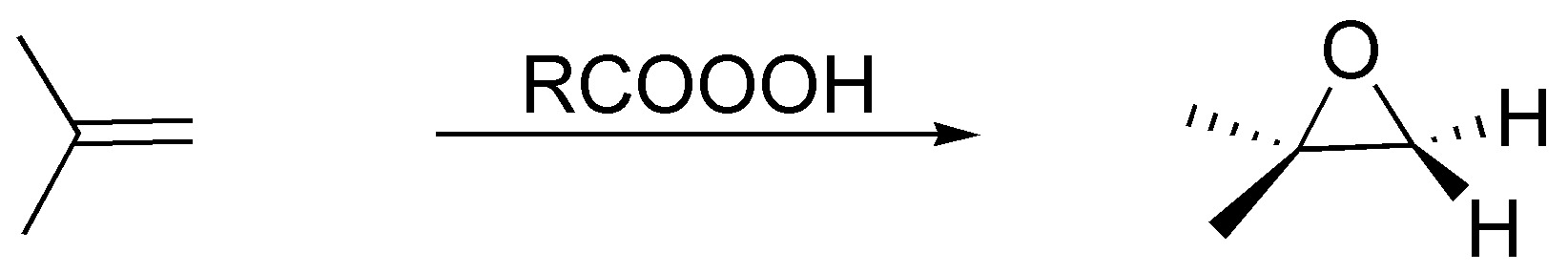
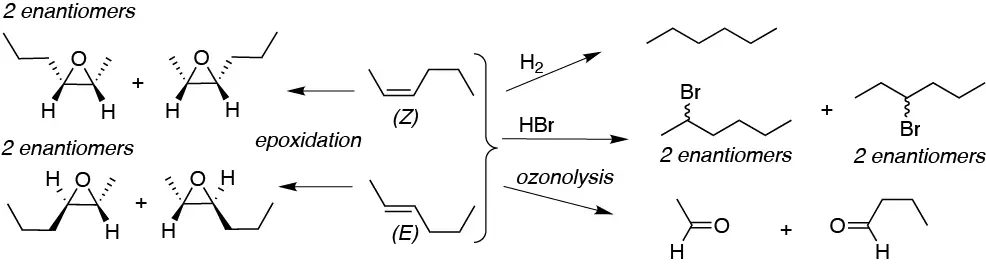
Solution 31:
As we can see, the only reaction that produces different products is epoxidation (stereospecific reaction). Hydrogenation, although normally also is, in this case produces hexane which is not chiral. Hydrohalogenation is only regioselective (not in this case) and produces the corresponding 2-bromo and 3-bromo hexanes as racemic mixtures.
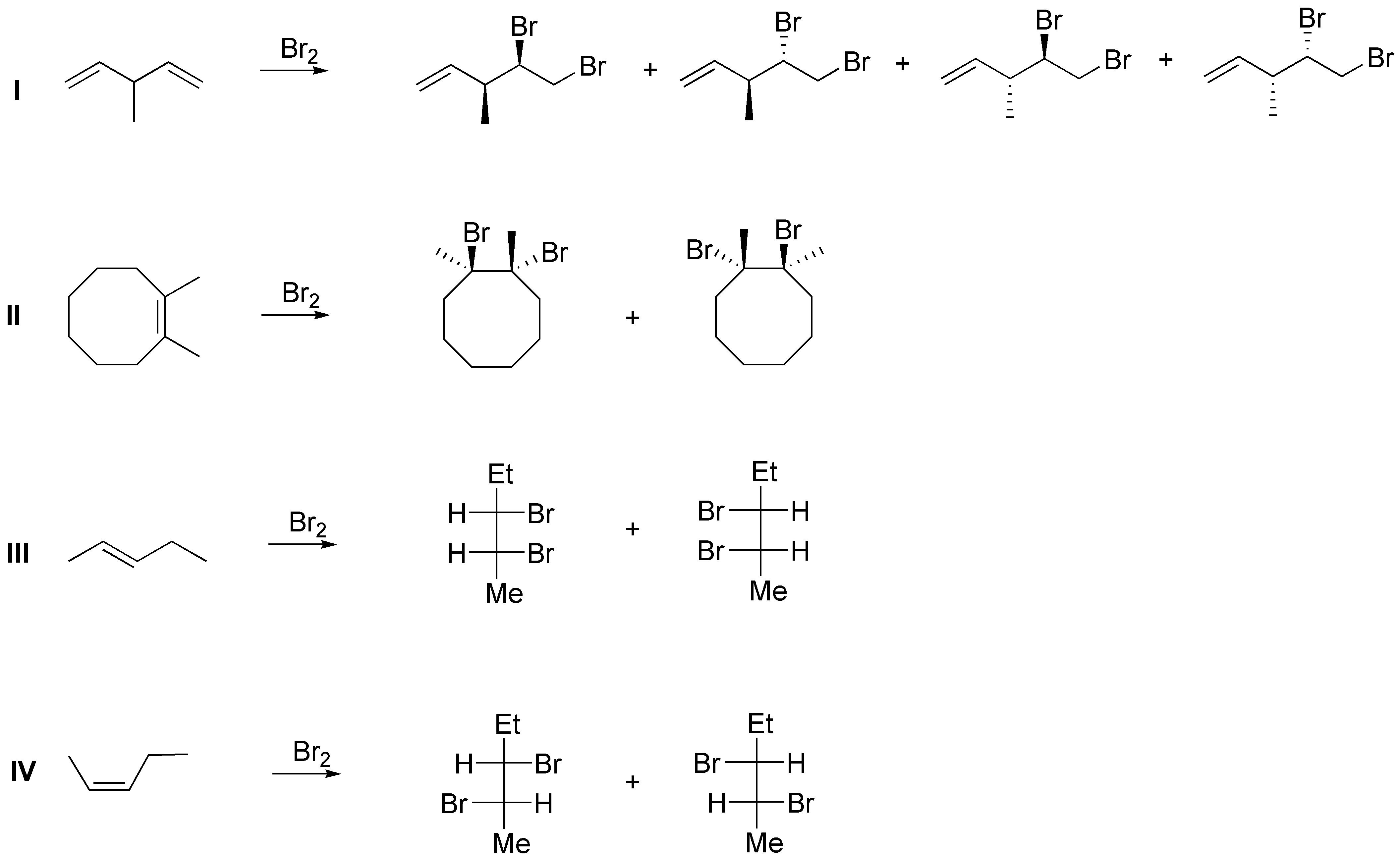
Solution 32:
The addition of bromine to an alkene is a stereospecific reaction, with anti stereoselectivity. Since the starting alkenes are not optically active (they are not chiral) the reactions must produce an optically inactive mixture: in the case I they will appear as a mixture of two pairs of enantiomers and in the remaining ones as a pair of enantiomers:
Solution 33:
The reconstruction of the starting alkene is achieved by simply replacing the carbonyl group by a double bond, the acid group by a CH= and the CO2 by a CH2=, thus we will have:
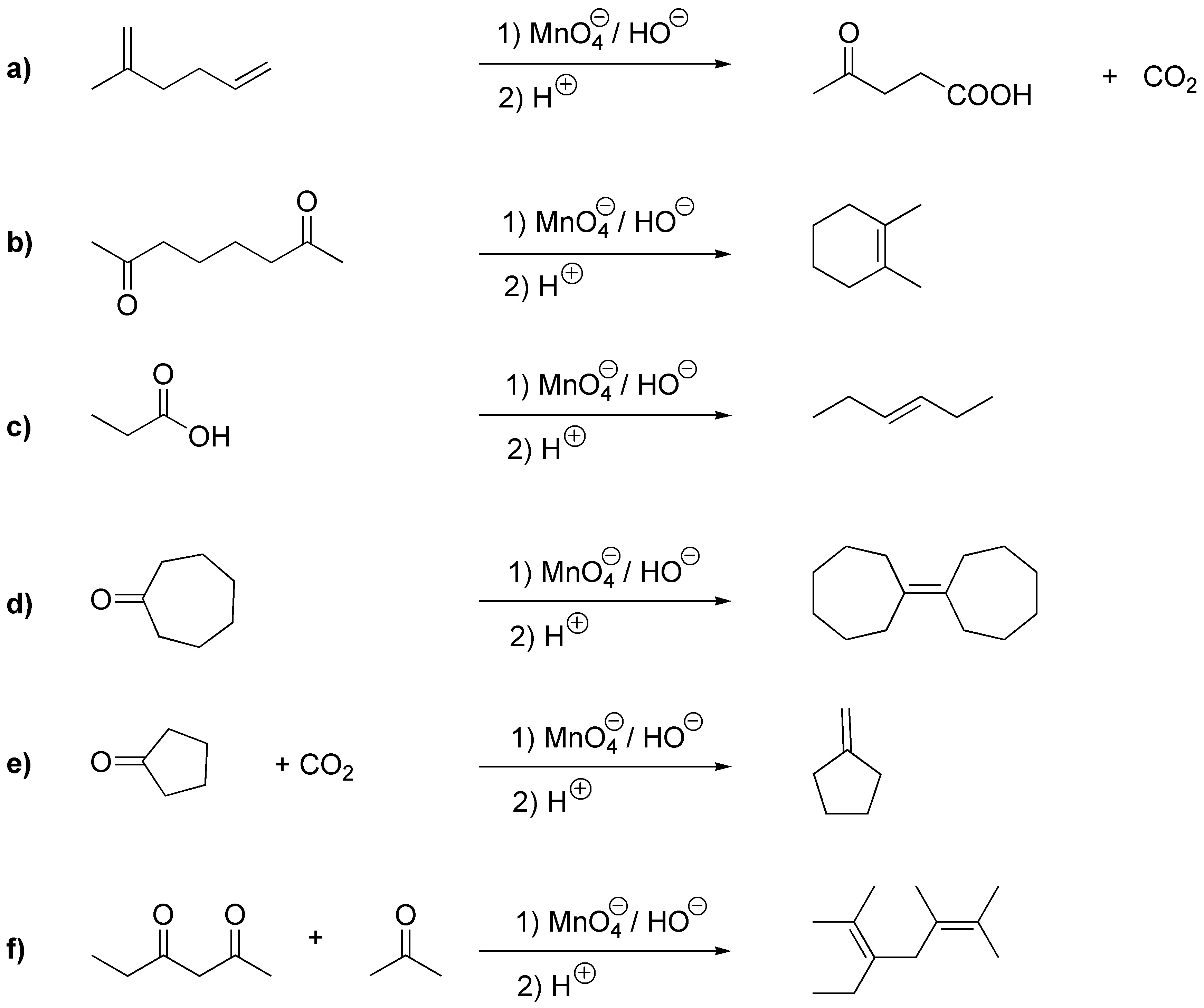
Solution 34:
The starting product must be a diene because it adds 4 hydrogen atoms. To determine the situation of the double bonds the solution is given by ozonolysis: the propanodial is the intermediate fragment of the structure and the ends will generate acetaldehyde and acetone. It is not possible to determine the stereochemistry of the product (E or Z) but the isomers obtained on reaction with HCl can be predicted.
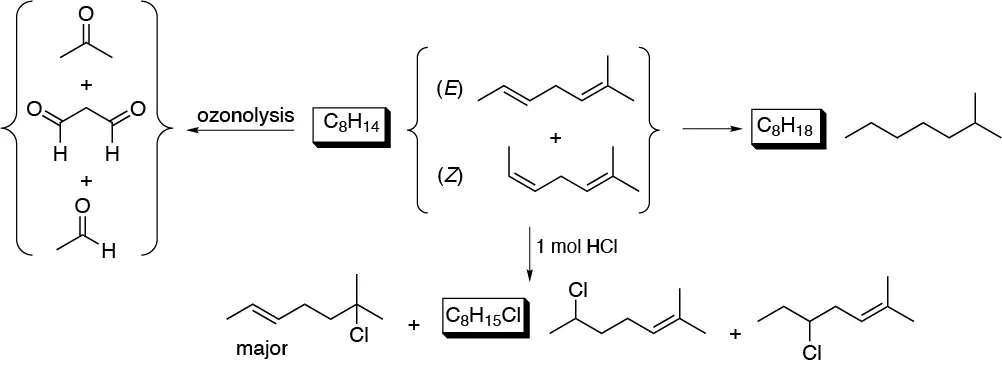
Solution 35:
Treatment with a mineral acid produces a carbocation that will react with another molecule of the alkene generating a new carbocation that loses a proton giving an alkene (in principle the most thermodynamically stable, i.e. the most substituted):
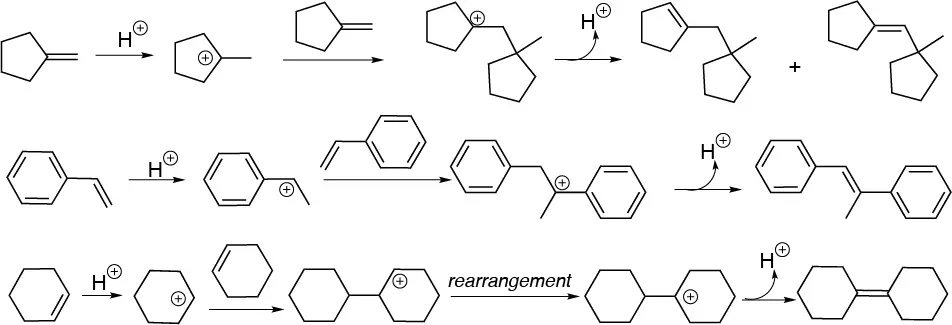
Solution 36:
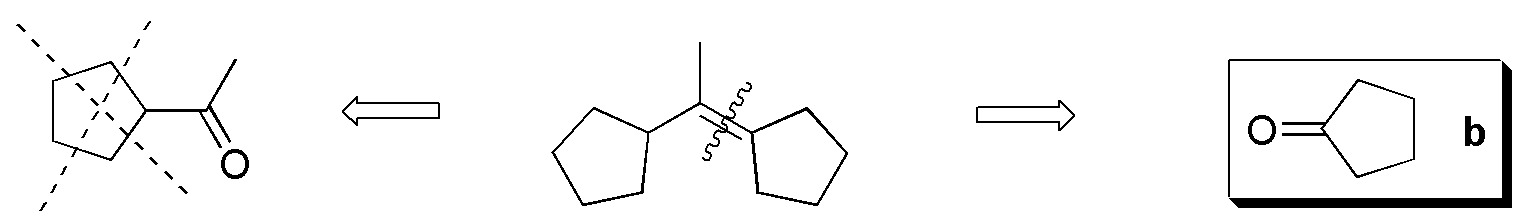
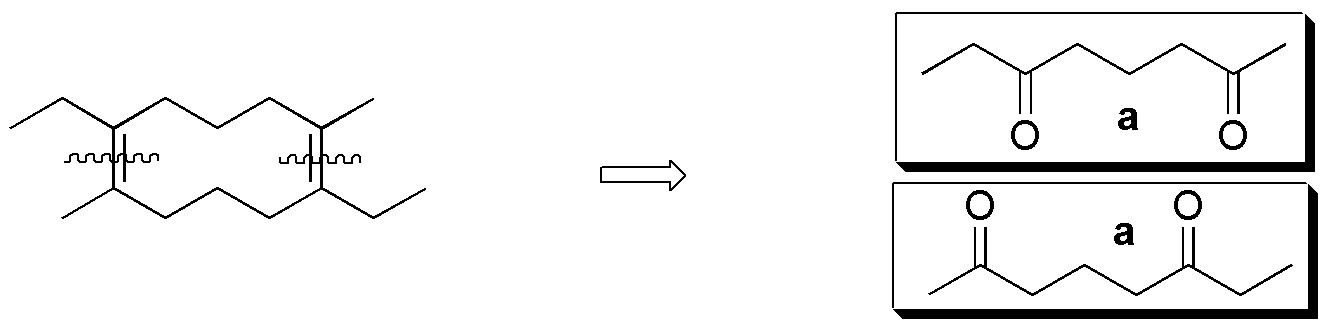
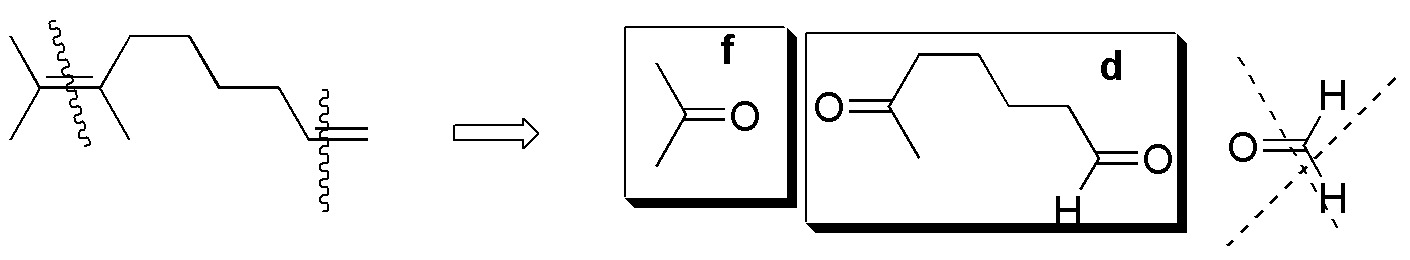
To perform this exercise in a systematic way it is better to consider each alkene separately and analyze one by one the possible fragments that can be obtained from each of them by ozonolysis reaction.
Compound I) The following fragments can be established:
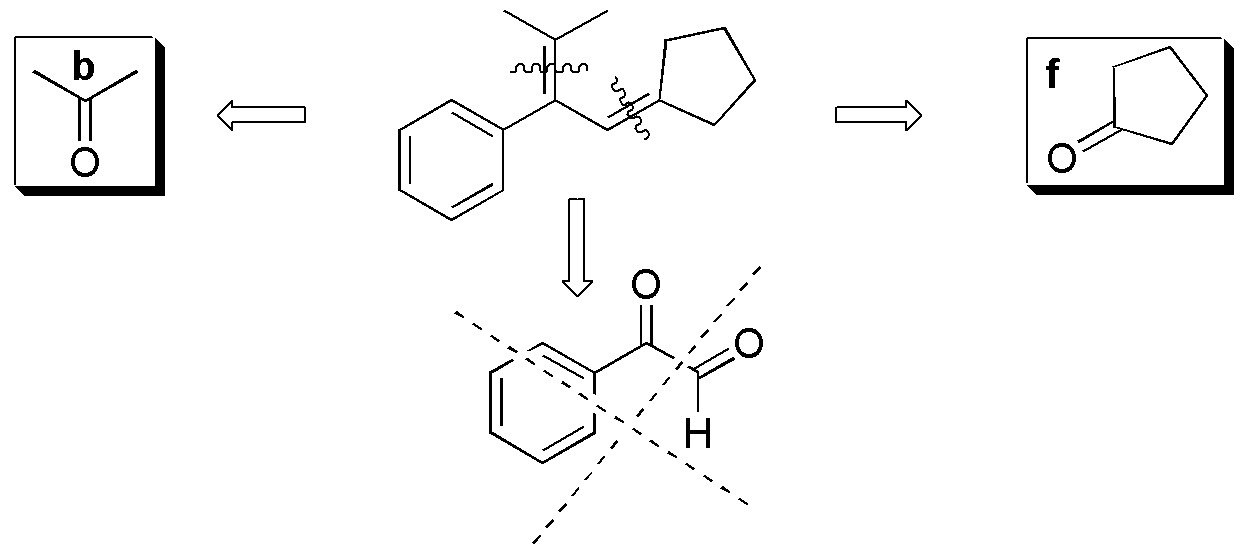
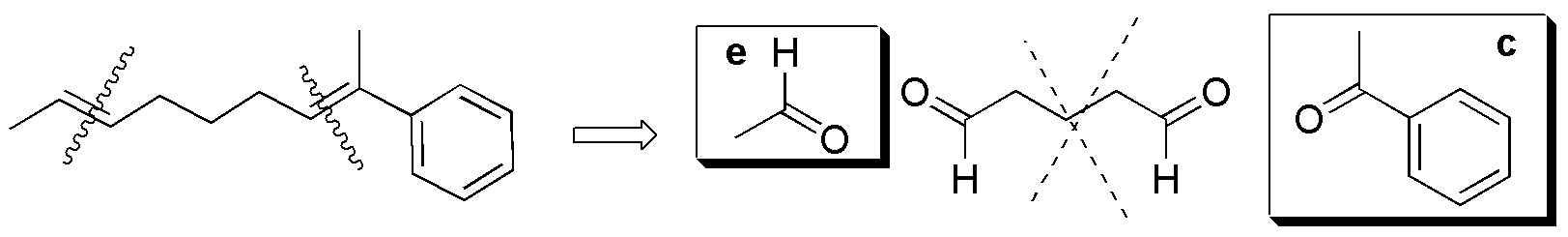
Compound II) Ozonolysis of II leads to the formation of 5-oxo-hexanal. None of the carbonyl compounds in figure (a-f) are obtained.
Compound III) However, ozonolysis of III yields 6-oxo-heptanal.
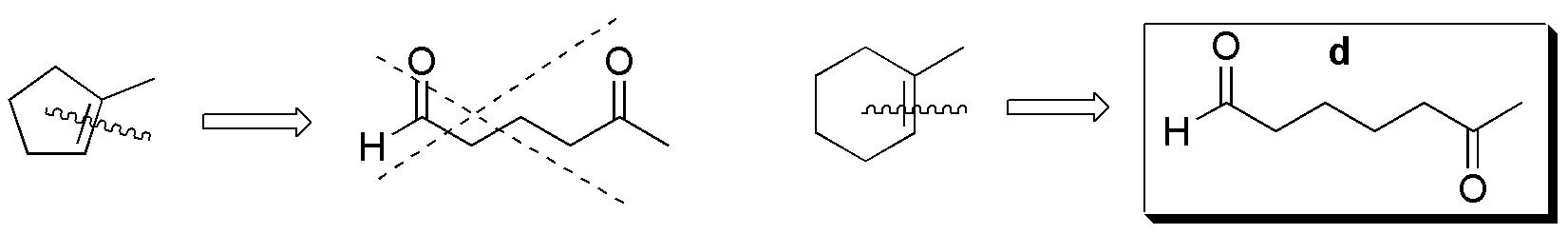
Compound IV) For IV, the possible fragments are:
Compounds V-VIII) For the remaining alkenes the results are given in the following scheme:
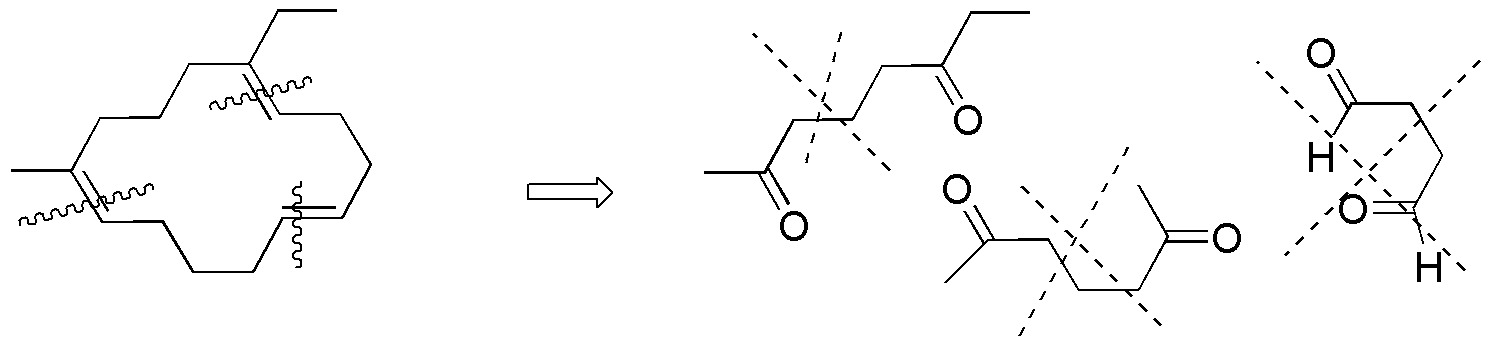
Solution 37:
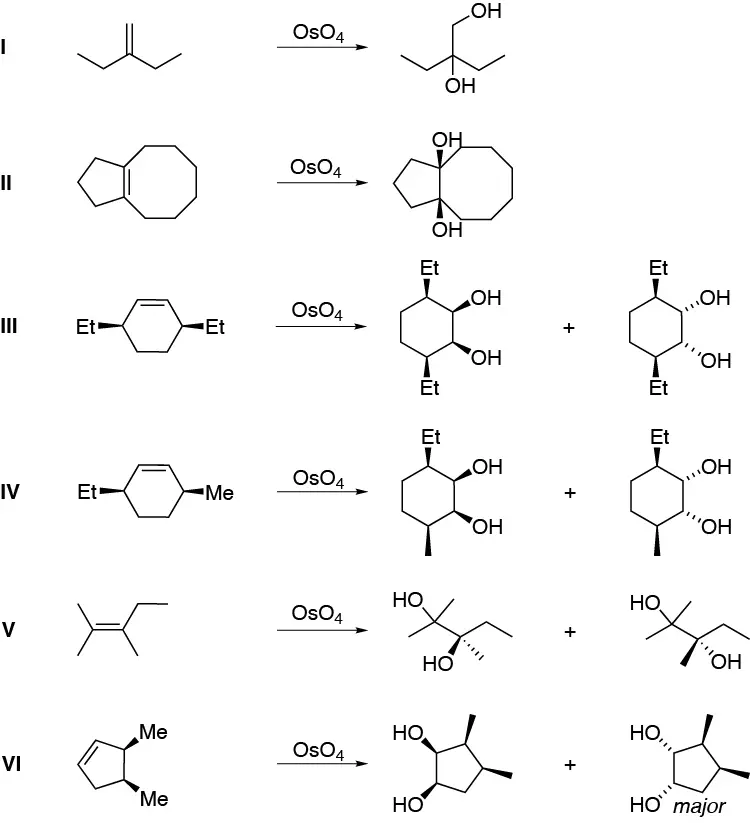
OsO4 is added to alkenes with syn stereochemistry giving rise to the formation of a cyclic osmiate, treatment of the same with H2S produces the corresponding dihydroxylated compound with syn stereochemistry:
Solution 38:
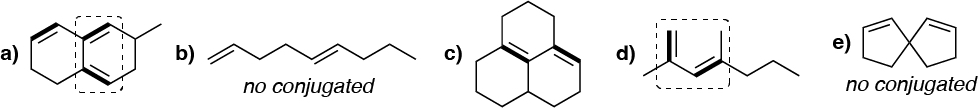
The conjugated dienes are highlighted with thick stroke and with dashed those suitable for the Diels-Alder reaction are indicated.
Solution 39:
The Diels-Alder reaction occurs between a conjugated diene (electron rich) with an alkene or alkyne (electron poor) to give a cyclohexene or cyclohexadiene respectively:
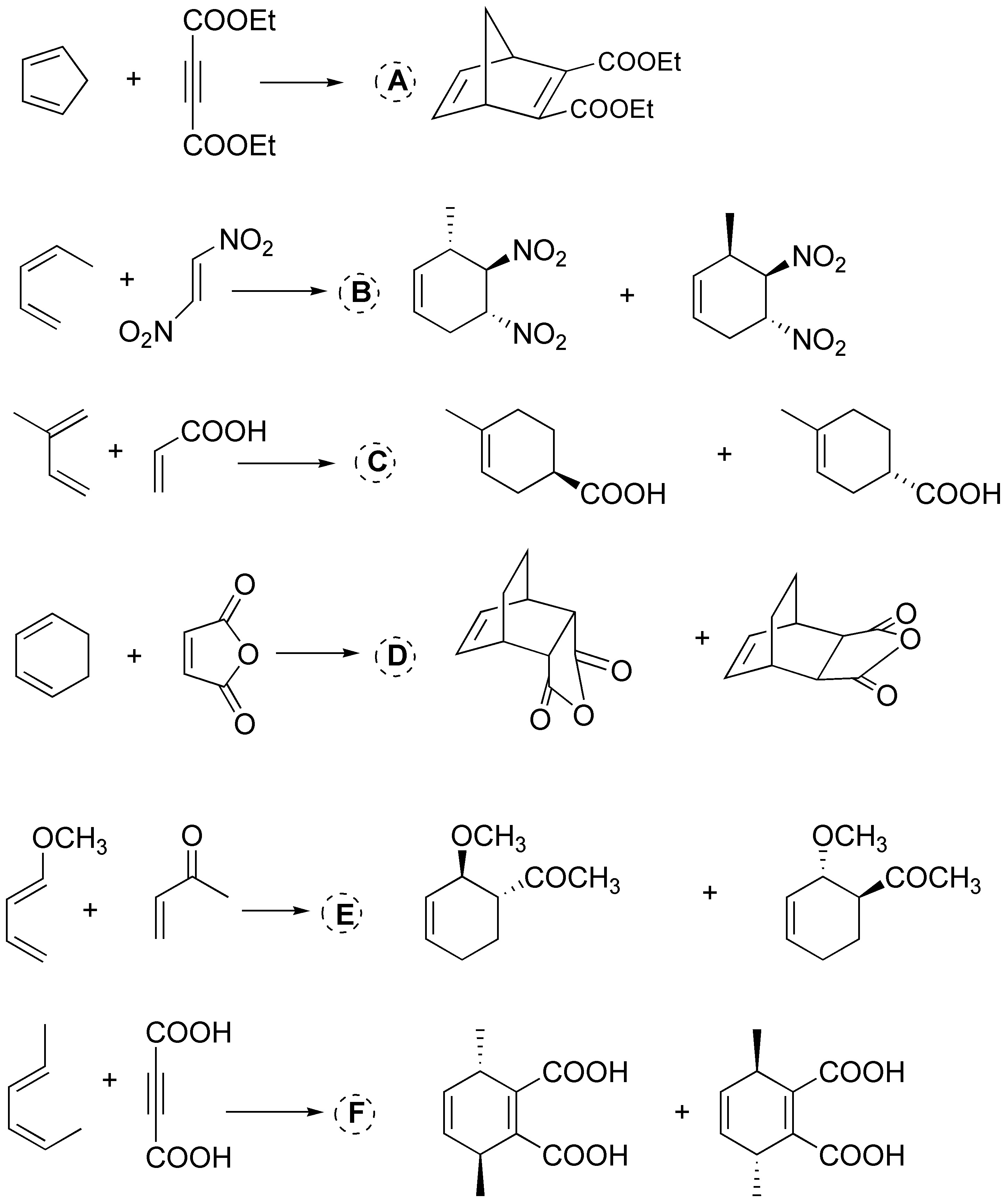
Solution 40:
Two addition products-1,4 (Z and E) are obtained and since the diene is not symmetric two addition products-1,2.
Solution 41:
Some characteristic reactions of dienes:
- a) Addition of one mole of bromine to a diene gives two products as a result of 1,2- or 1,4- addition (kinetic control or thermodynamic control)
- b) and c) illustrate the differences in addition to dienes at low temperature the kinetic control product predominates (1,2- addition) while at higher thermperatures the more stable product predominates (thermodynamic control)
- d) the Diels-Alder reaction with an alkyne will yield cyclohexa-1,4-dien carboxylic acid.
- e) hydrogenation gives an alkane.
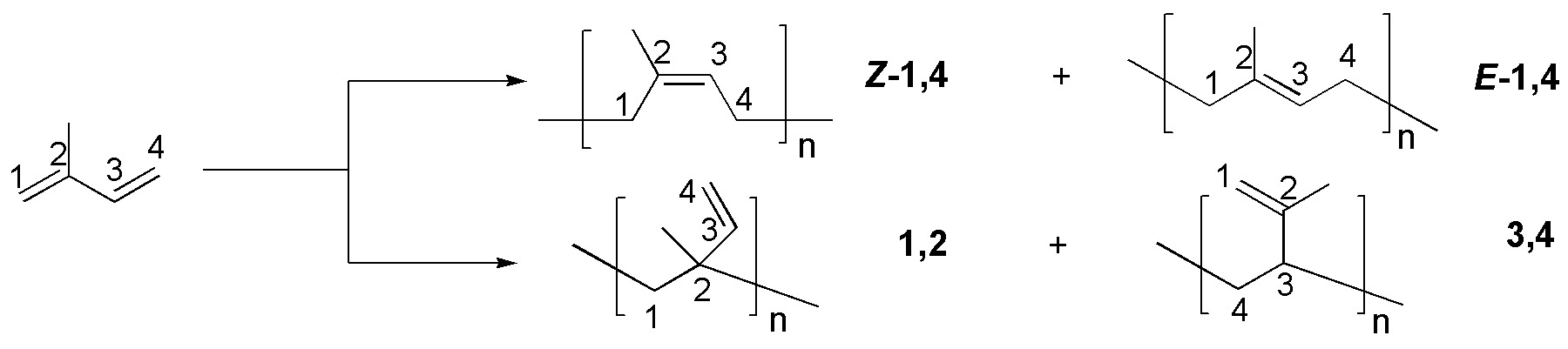
Solution 42:
Some characteristic reactions of dienes:
Solution 43:
Catalytic hydrogenation of an alkene is a normally stereospecific reaction with stereochemistry syn (two different stereoisomers produce different stereoisomers) but in these cases it is not as they produce achiral products.
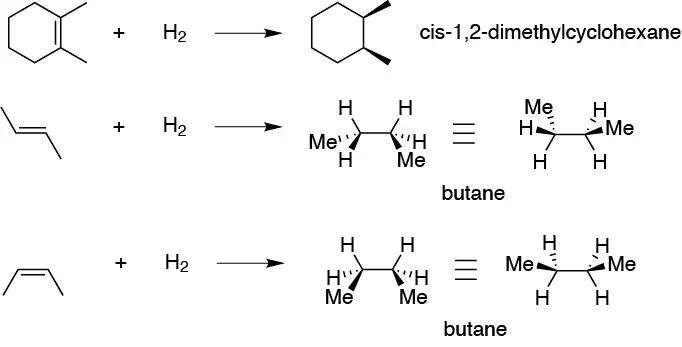
Solution 44:
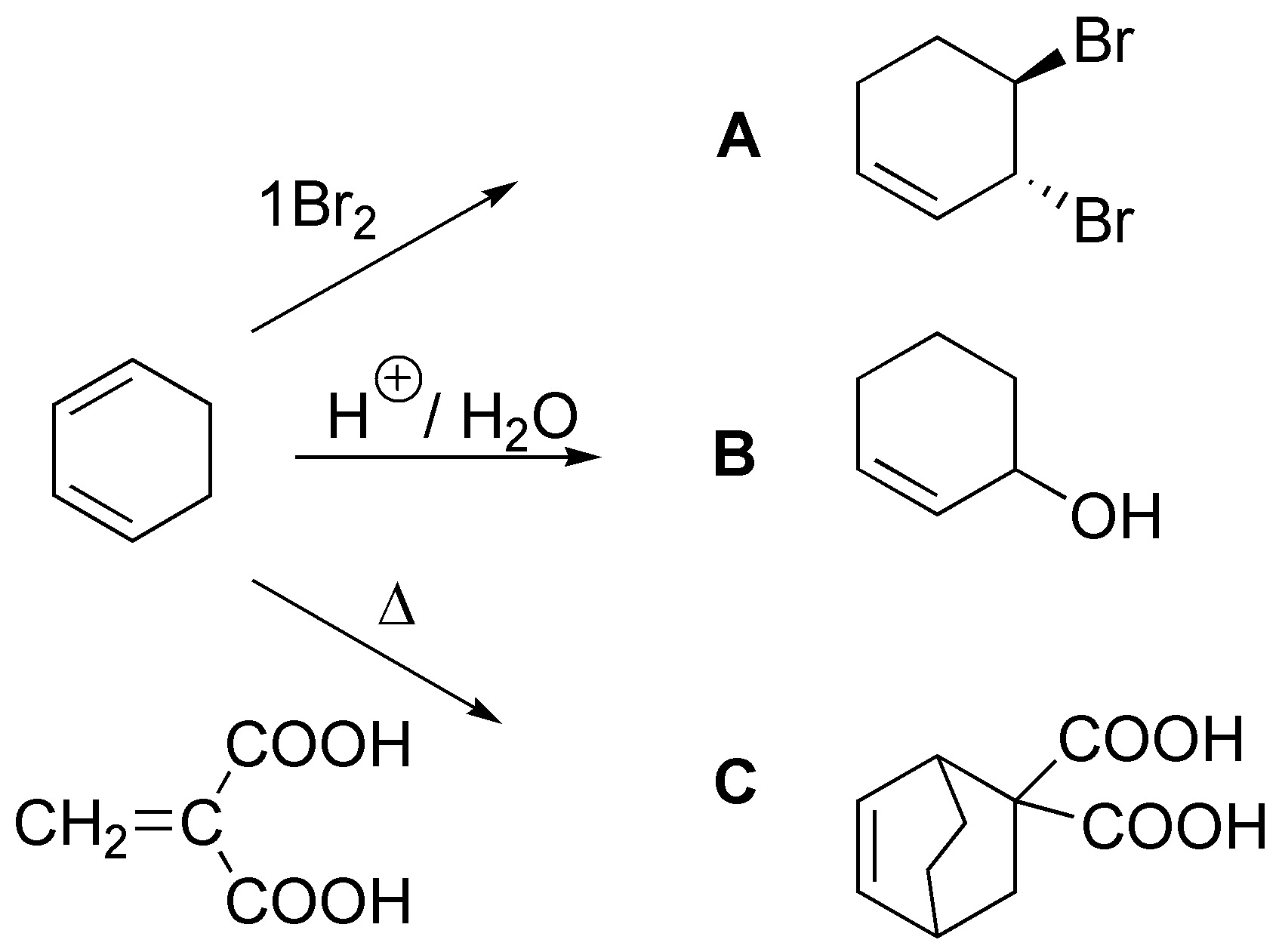
The addition of halogens is an anti addition, thus trans-1,2-dibromocyclohexane will be produced as a mixture of enantiomers:

Solution 45:
Addition of chlorine in the presence of water would result in the formation of a chlorohydrin (chloroalcohol) and has an anti stereochemistry.

Solution 46:
The reaction starts with the addition of a proton to the alkene generating a carbocation which is trapped by water giving a hydronium ion which loses a proton giving the corresponding alcohol:
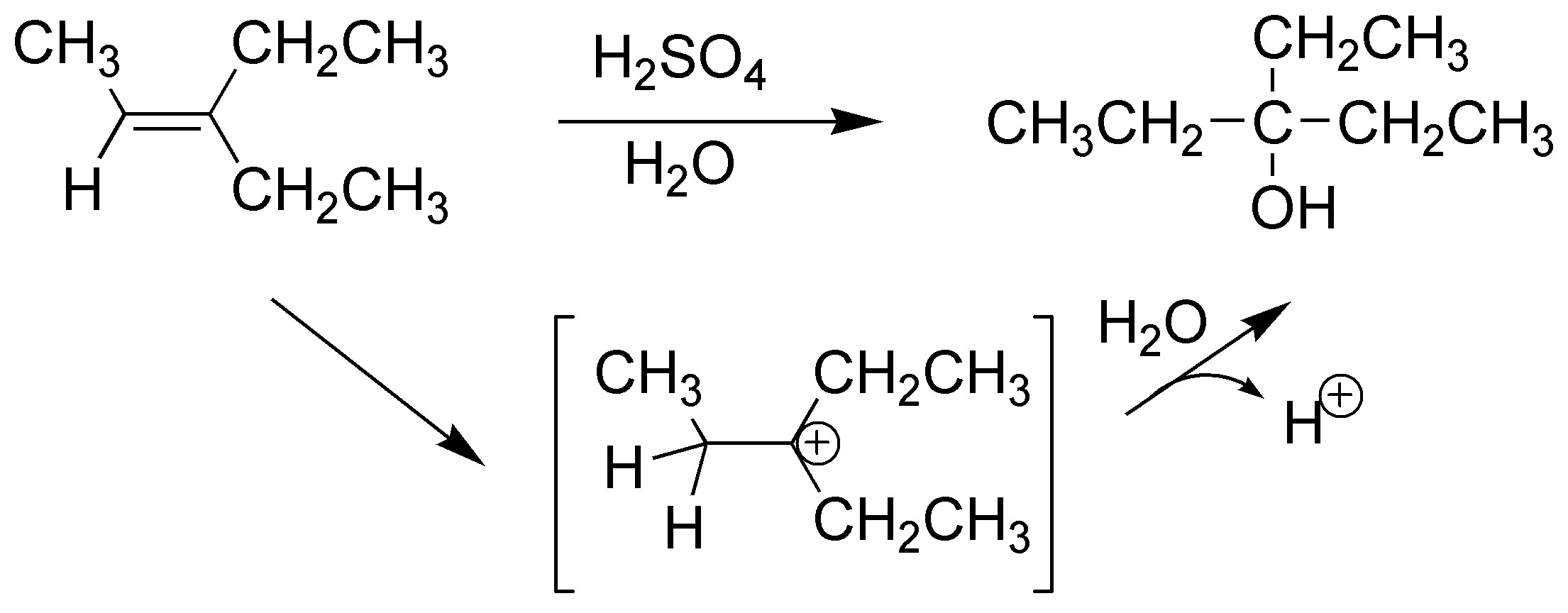
Solution 47:
Alkenes with diazomethane give rise to the formation of cyclopropanes by addition of the diradical carbene, while epoxidation produces epoxides (oxacyclopropanes), both reactions being stereospecific:
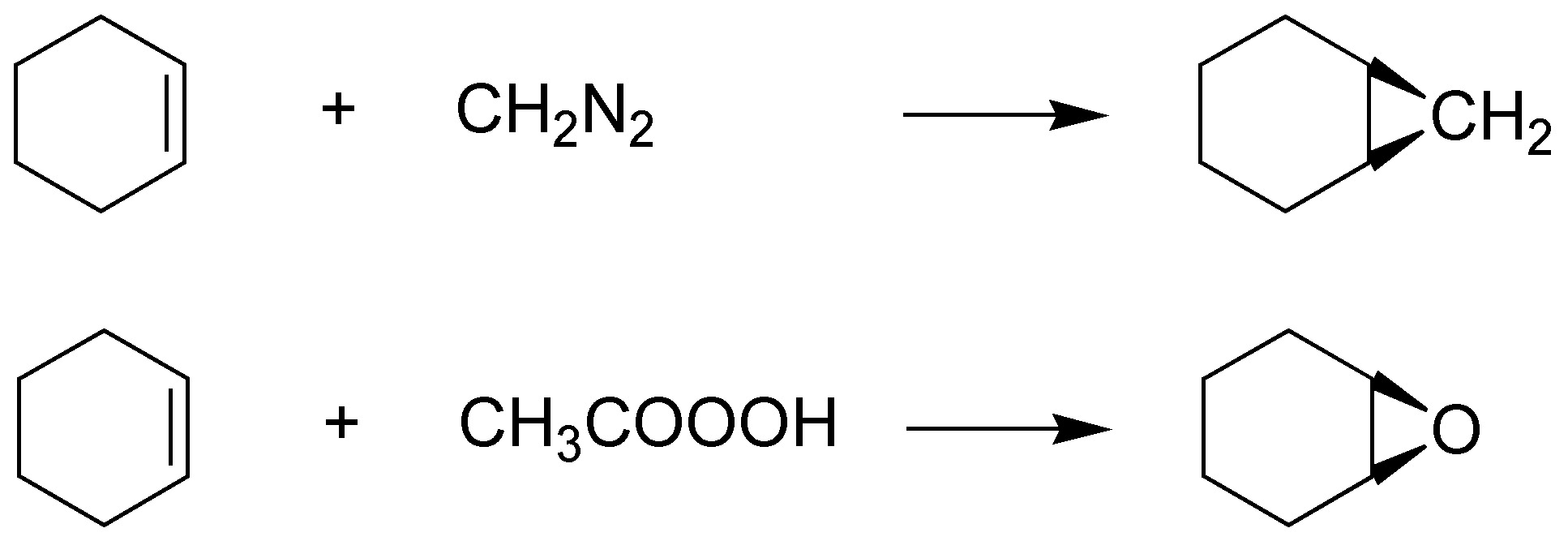
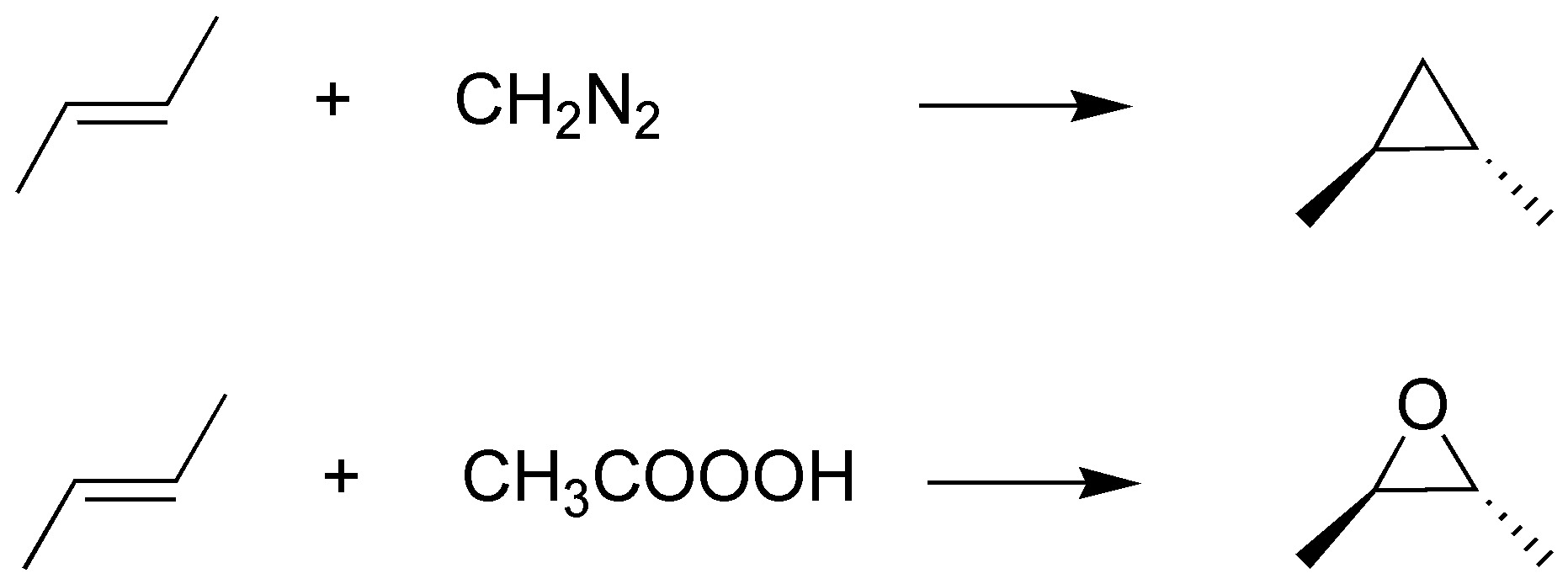
Solution 48:
Regrouping occurs with migration of the methyl group.
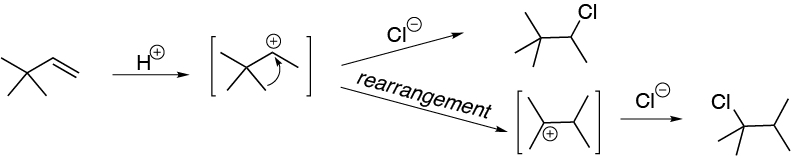
Solution 49:
As a result of the Diels-Alder reaction, cyclohexenes (if the dienophile is an alkene) or 1,4-cyclohexadienes (if the dienophile is an alkyne) are obtained, the reaction is stereoselective (endo stereoselectivity) and stereospecific (the stereochemistry of the starting alkene is preserved):
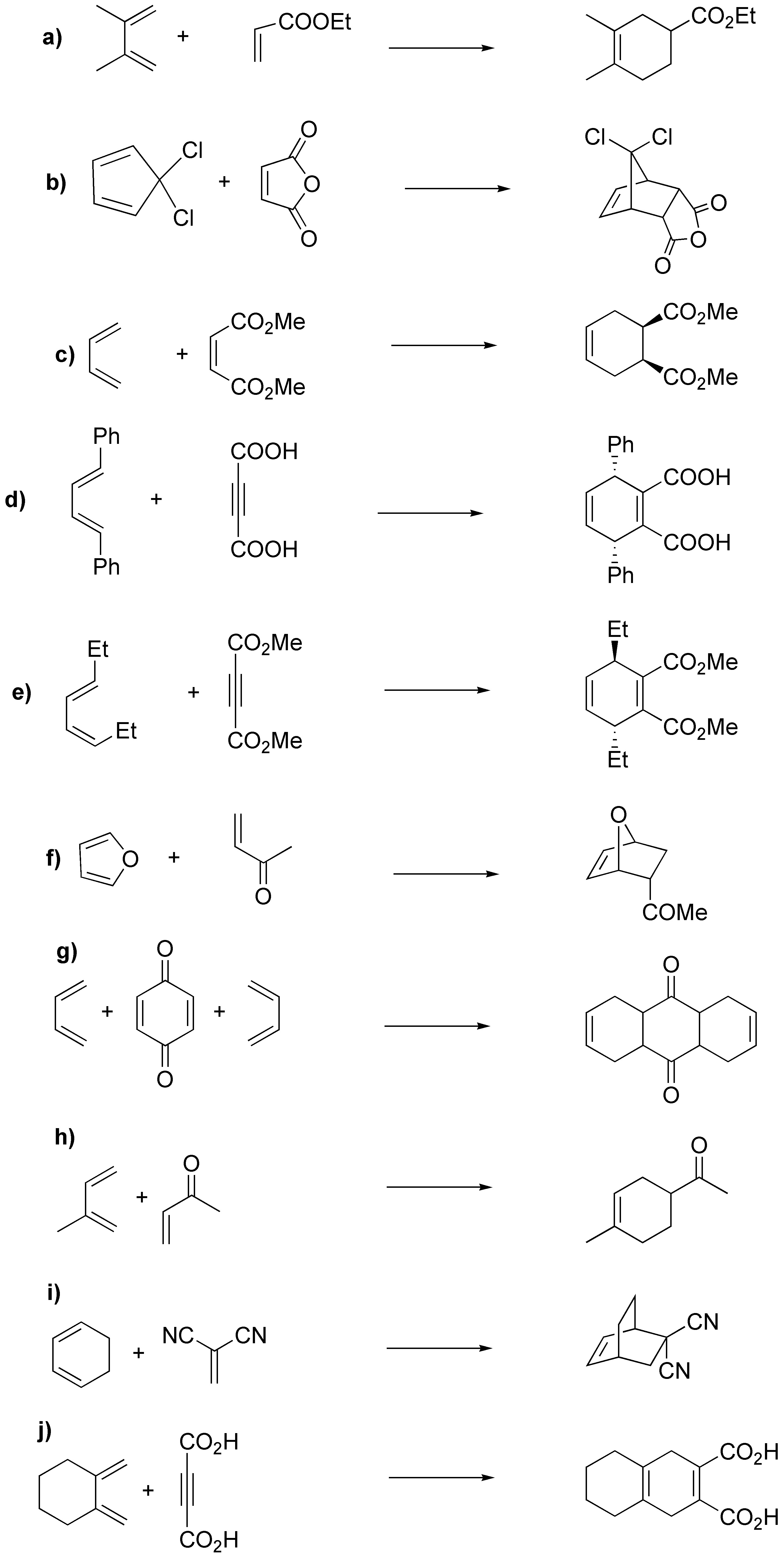
Solution 50:
As can be seen in the figure the dienophile and dienophile systems are identified and presented to give the corresponding intramolecular Diels-Alder reaction:
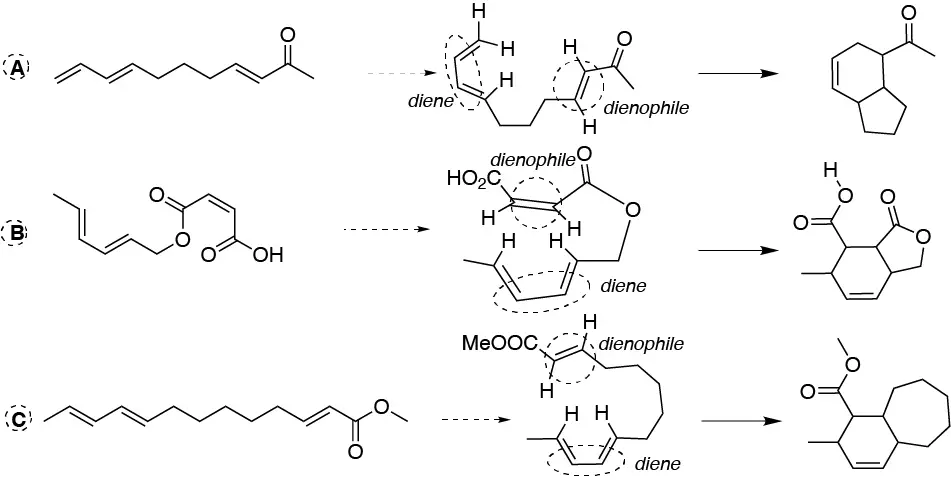
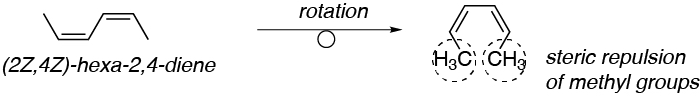
Solution 51:
As can be seen in the figure the conformation to be acquired by the diene is poorly favored by the steric repulsion of the methyls attached to the conjugated double bond system, so that the (Z,Z)-hexa-2,4-diene will be poorly reactive against Diels-Alder.