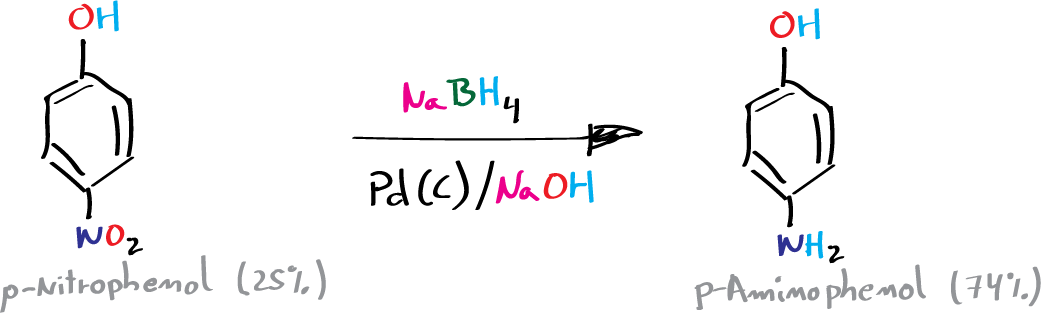Written by J.A Dobado | Last Updated on May 2, 2024
Objective
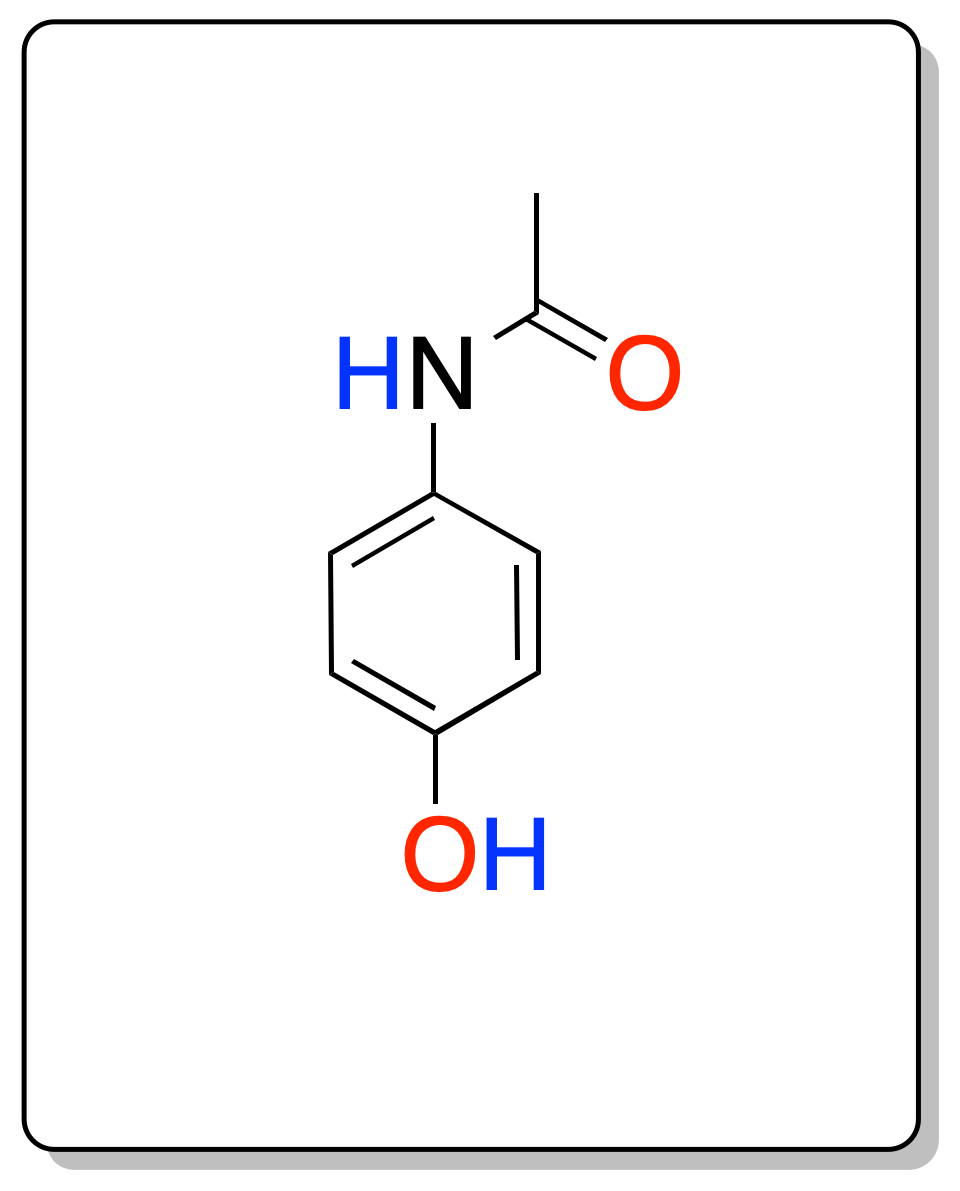
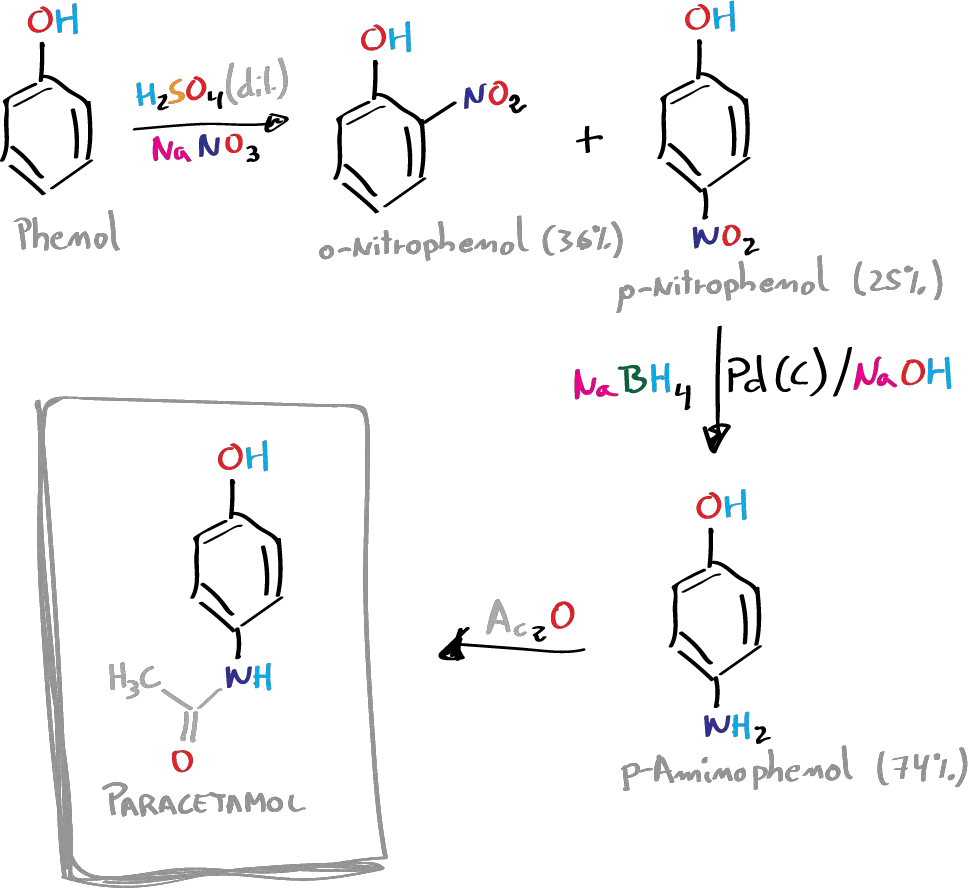
In this experiment, the three-step synthesis of paracetamol (p-acetamidophenol) from phenol is carried out.
| 3D structure |
Background
paracetamol (p-acetaminophenol) is a crystalline solid with a melting point of 169-171 ºC that is used as an analgesic and antipyretic, although it has no significant anti-inflammatory action. Commercially it appears under different names: Commercially it appears under various names Acertol®, Actron®, Antidol®, Apiretal®, Bandol®, Calmanticold®, Cupanol®, Dafalgan®, Dolgesic®, Dolostop®, Duorol®, Efferalgan®, Febrectal®, Gelocatil®, Melabon infantil®, Panadol®, Pediapirin®, Perfalgan®, Resakal®, Sinmol®, Temperal®, Termalgin®, Tylenol®. For example, in 1955 it was put on sale in the USA under the name Tylenol, in 1956, in the UK under the name Panadol. It is a drug (non-opioid analgesic and non-steroidal anti-inflammatory drug) that is on the WHO list of essential medicines.
The synthesis of paracetamol was first performed by J. von Mering in 1893 although it was not commercialized until 1953, being considered since then as a safer active ingredient than aspirin, especially for children and patients with stomach ulcer.
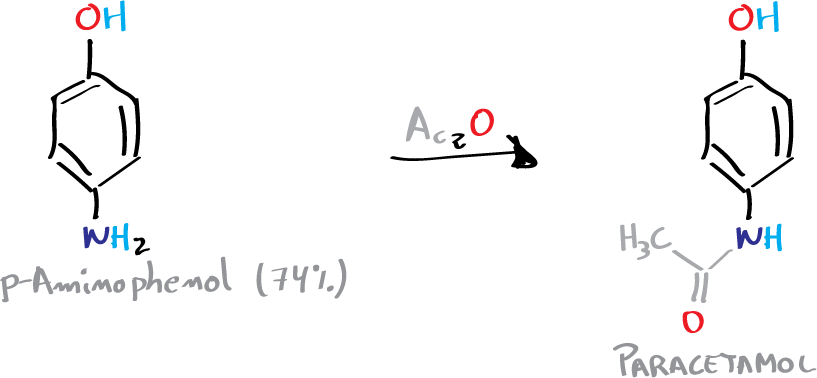
Paracetamol can be synthesized in three steps from phenol. In the first stage, phenol is nitrated and the isomers obtained are separated. In the second stage, the nitro group is reduced to the amino group, and in the third stage, the amino group is acetylated with acetic anhydride selectively against the phenolic -OH group.
Experimental procedure
| DANGER! “Carry out all experiments in fume cupboard”. |
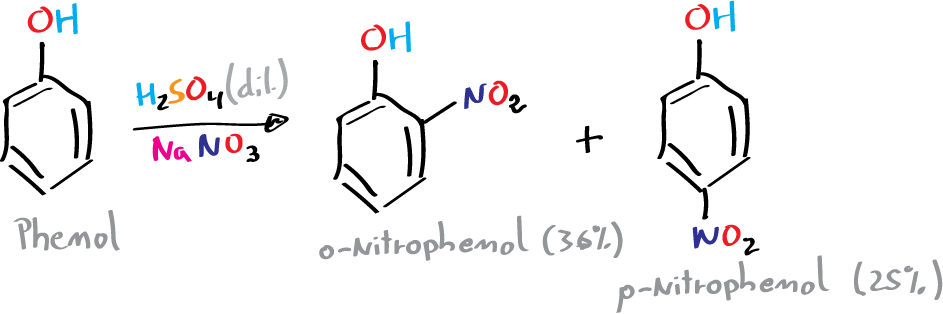
A) Nitration of phenol: synthesis of p-nitrophenol
To a 250 ml flask, cooled with an ice bath, 15 g of sodium nitrate and 40 ml of water are added. The mixture is stirred until the solid is completely dissolved, and then 25 g (13.6 ml) of concentrated H2SO4 is added little by little. Then, 9.4 g of phenol are slowly added with the help of a spatula, so that the temperature of the mixture does not exceed 20 °C, which will take about 20 min.
After finishing of the addition, the reaction is stirred for 2 h at room temperature. Under these conditions a mixture of the o– and p-nitrophenol is produced.
With the aid of a Pasteur pipette, the supernatant liquid is removed, 30 ml of water is added and a setup is made for a steam entrainment distillation with internal steam source. The mixture is heated, adding water from the addition funnel at the same rate as the distillation of the vapors, until the product (2-nitrophenol) stops distilling.
When the mixture remaining in the flask is cooled, crystallization of the other isomer (4-nitrophenol) occurs, which can be recrystallized using an HCl solution of concentration 0.5 moles / 100 ml.
B) Reduction of the nitro group to amino: synthesis of p-aminophenol
To an Erlenmeyer of 100 ml is added 4 g of NaOH, and 10 ml of deionized water. After the base has dissolved and the solution has cooled to room temperature, 0.56 g (14.7 mmol) of NaBH4 and 50 mg of 5 % Pd(C) are added.
The mixture is cooled in an ice/salt bath (-12 °C) and once cooled, 1 g (7. 2 mmol) of p-nitrophenol with magnetic stirring for 30 min, keeping the reaction temperature around 15 °C.
Check the temperature inside the Erlenmeyer flask with a thermometer without the stir bar hitting the bulb.
Once the addition is finished, the agitation is maintained at room temperature for another 15 min.
The reaction crude is then acidified with 6 M concentration HCl.
The catalyst is filtered under vacuum and the filtrate is pH adjusted to 8 by addition of NaHCO3 in small portions. The precipitate is filtered under vacuum, dried and weighed for use in the next step (estimated yield 75 %).
C) Synthesis of paracetamol: amide formation
In an Erlenmeyer containing 3.3 g of p-aminophenol and 9 ml of water, 3.6 ml of acetic anhydride are added dropwise with caution, stirring the mixture constantly. It is then heated in a water bath at 60 °C until complete dissolution of the solid.
Stirring is maintained for an additional 10 min and then the solution is cooled in an ice bath until the appearance of a slightly pink crystalline product. The crystals appearing in the reaction crude are filtered under vacuum in a Büchner.
The final product can be analyzed using thin-layer chromatography (TLC). The Rf value of the product was found to be 0.50 and mesurement of melting point 169-171 ºC.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| Benzoic acid | 122.12 | 125 | 249 | 1.08 |
| Phenol | 94.11 | 40-42 | 182 | 1.07 |
| o-Nitrophenol | 139.11 | 45 | 214 | - |
| p-Acetamidophenol | 151.16 | 168-172 | - | - |
| p-Nitrophenol | 139.11 | 110-115 | 279 | 1.480 |
| 4-Aminophenol | 109.13 | 185-189 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic anhydride |    |
| Benzoic acid |   |
| Phenol |    |
| o-Nitrophenol |  |
| p-Acetamidophenol |  |
| p-Nitrophenol |   |
| 4-Aminophenol |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| Benzoic acid | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
| Phenol | ISWSIDIOOBJBQZ-UHFFFAOYSA-N |
| o-Nitrophenol | IQUPABOKLQSFBK-UHFFFAOYSA-N |
| p-Acetamidophenol | RZVAJINKPMORJF-UHFFFAOYSA-N |
| p-Nitrophenol | BTJIUGUIPKRLHP-UHFFFAOYSA-N |
| 4-Aminophenol | PLIKAWJENQZMHA-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- F. Ellis, C. Osborne, and M. J. Pack, Paracetamol: A Curriculum Resource, Royal Society of Chemistry, 2002.