Go to the page with the solutions to the problems.
Alkynes – list of problems
Problem 1)
Complete the following reaction:
pent-2-ino + H2 (Lindlar’s catalyst) →
Problem 2)
Predict the result of the following transformation:
hex-3-ino + H2 (Na / NH3) →
Problem 3)
State the final product obtained by addition of one mole of HCl to but-2-ino.
Problem 4)
In the above case, what will be the result if an excess of HCl is used?
Problem 5)
Complete the following reaction, justifying the result.
3,3-dimethylbut-1-ino + 2 HBr →
Problem 6)
What will be the result of the addition of one mole of HBr over 3,3-dimethylbut-1-ino in the presence of peroxides? Describe the mechanism that takes place in such a transformation.
Problem 7)
Describe what will be the result of the addition of one mole and two moles of Cl2 independently over hex-3-ino in dichloromethane.
Problem 8)
The acid-catalyzed addition of water over hex-3-ino leads to the formation of a carbonyl compound. Justify this result and compare it with the product obtained under the same conditions with hex-3-ene.
Problem 9)
The addition reaction of water on but-1-ino is carried out by H2SO4/HgSO4 catalysis. Propose a mechanism for this transformation.
Problem 10)
Indicate what will be the result of the reaction of borane on but-1-ino followed by treatment with hydrogen peroxide in basic medium.
Problem 11)
Predict the major product that would be obtained from but-1-ino in each of the following transformations, indicating if applicable, the regio- and stereo-chemistry of the process, as well as the reagents and reaction conditions:
- a) addition of 1 mol of halogen
- b) hydroboration/oxidation
- c) catalytic hydrogenation
- d) reduction with metals
- e) oxidation with permanganate
- f) addition of HX
Problem 12)
Repeat the same previous problem 11) starting from but-2-ino, proposing a mechanism for process c).
Problem 13)
Complete the following scheme (A-H):
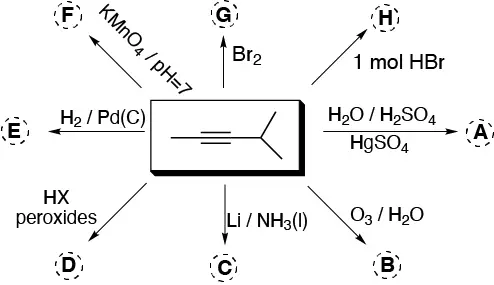
Problem 14)
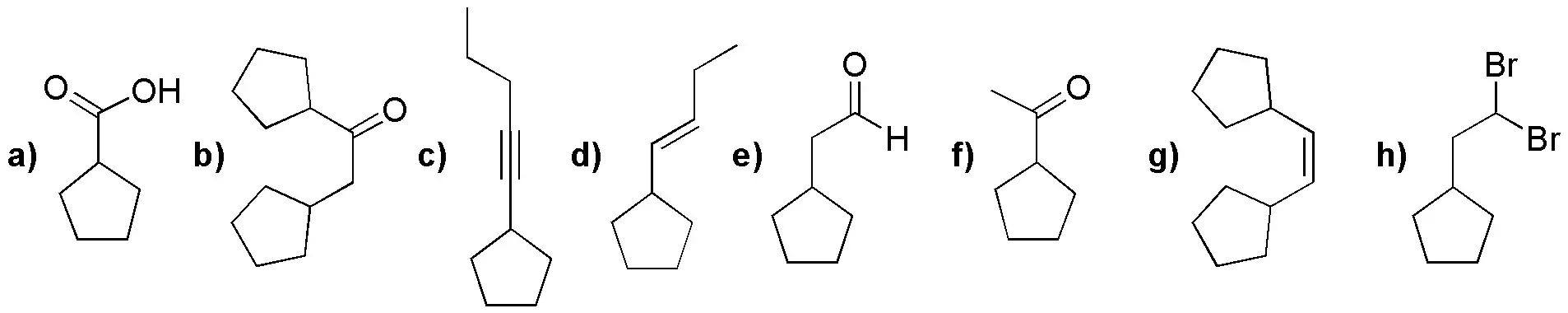
Starting from alkynes (I-III), choose the most appropriate reaction conditions to obtain the products (a-h) given below:
![]()
Problem 15)
The reactions given in the following scheme are incorrect. Explain what the error consists of, and what would be the product obtained.
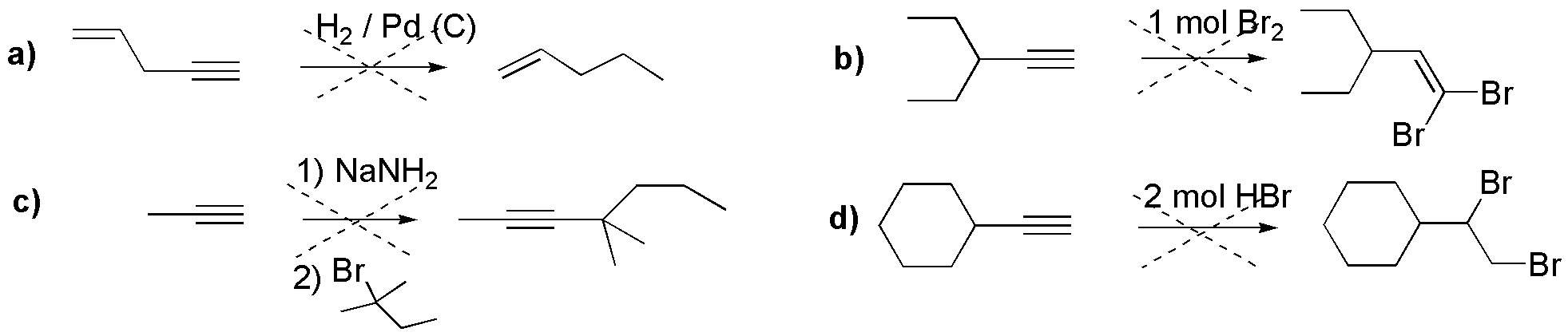
Problem 16)
Indicate, explaining the possible mechanism, the result of the addition of one mole of bromine in water to 1-butene.
Problem 17)
Complete the following scheme (A-I):
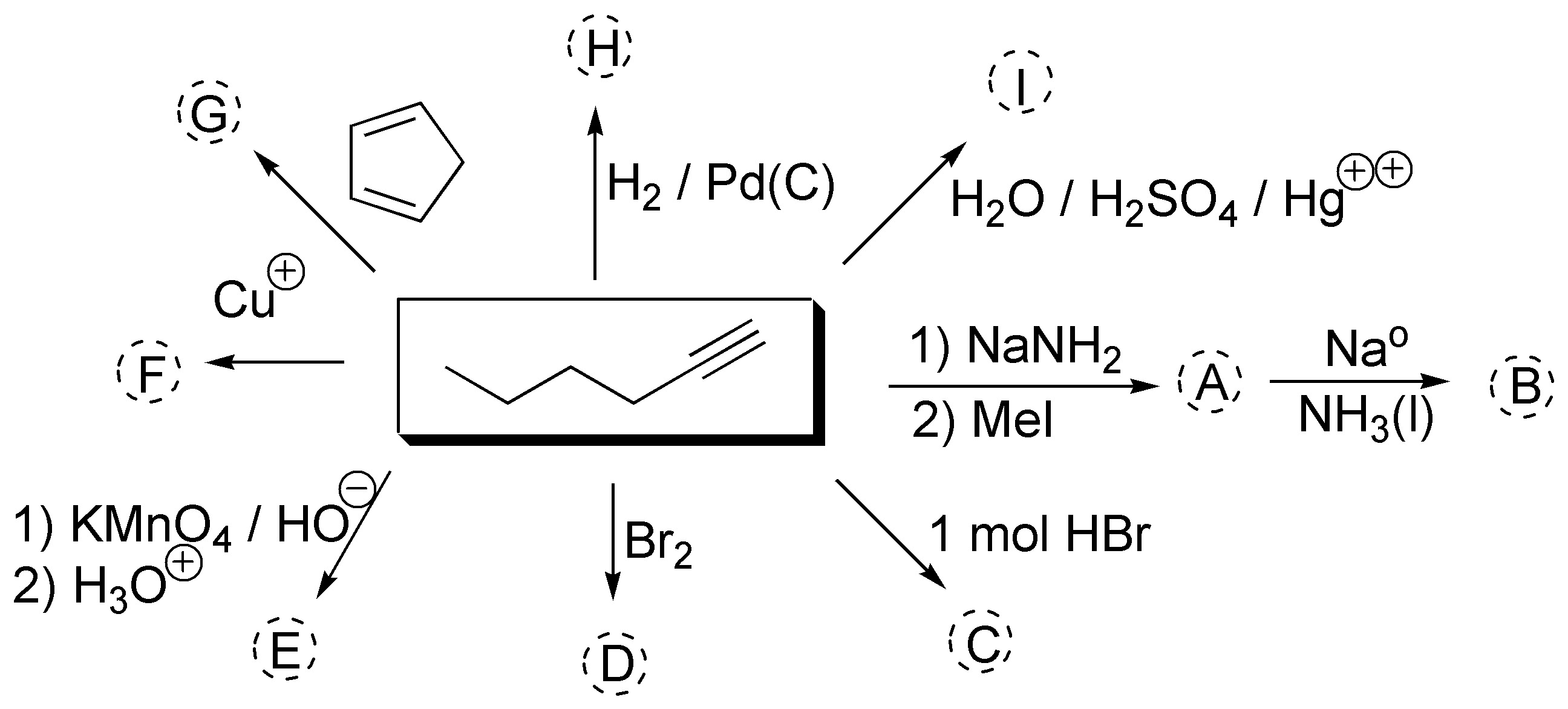
Problem 18)
Complete the following reactions (a-f) indicating the appropriate reagent (A-F) in each case.
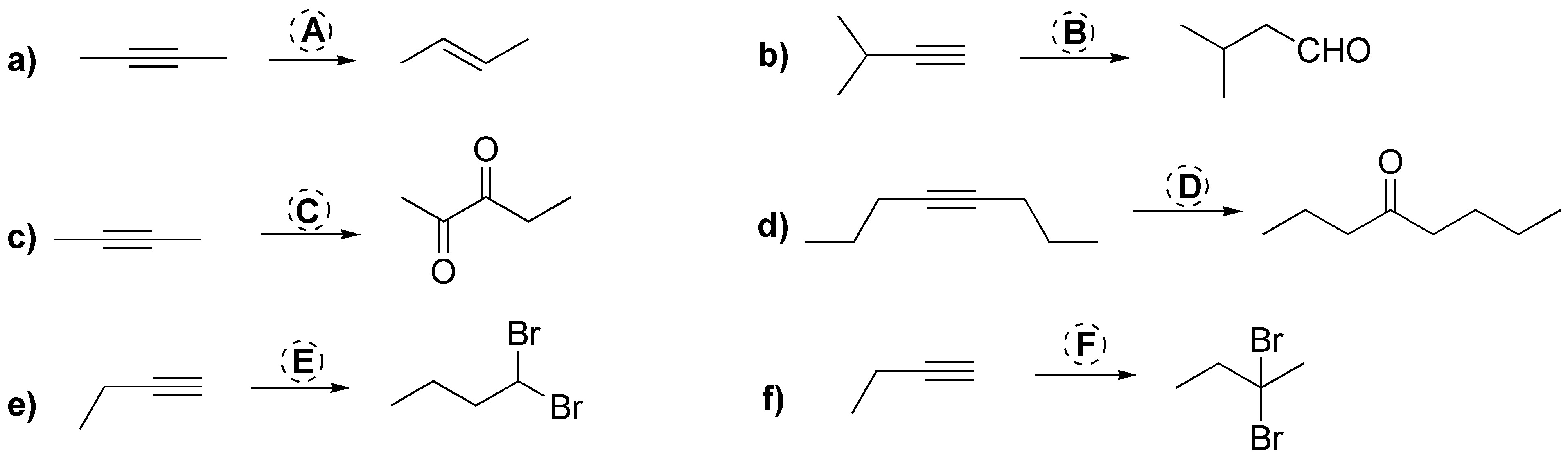
Problem 19)
Suggest an explanation for the increasing order of acidity alkyne > alkene > alkane.
Problem 20)
Identify the fragments obtained by ozonolysis of the following alkynes:
![]()
Problem 21)
Complete the following reactions:
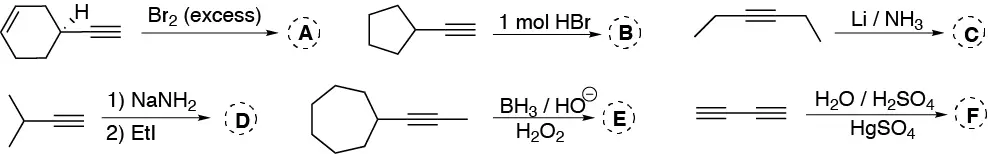
Problem 22)
Of the following bases (a-e), choose the one most suitable for the preparation of acetylides:
- a) NaOH
- b) NaH
- c) NaMeO
- d) NaNH2
- e) NaHCO3
Problem 23)
Choose, from the following list, the most suitable reagent for the transformations (A-F) given:
- 1 mol Br2
- 2 moles Br2
- 2 moles HBr
- BH3 / HO– / H2O2
- H2 / Pt
- H2 / Lindlar catalyst
- H2O / H2SO4/ HgSO4
- HBr / peroxides
- KMnO4 / HO– / H3O+
- KMnO4 / pH=7
- Li / NH3(l)
- O3
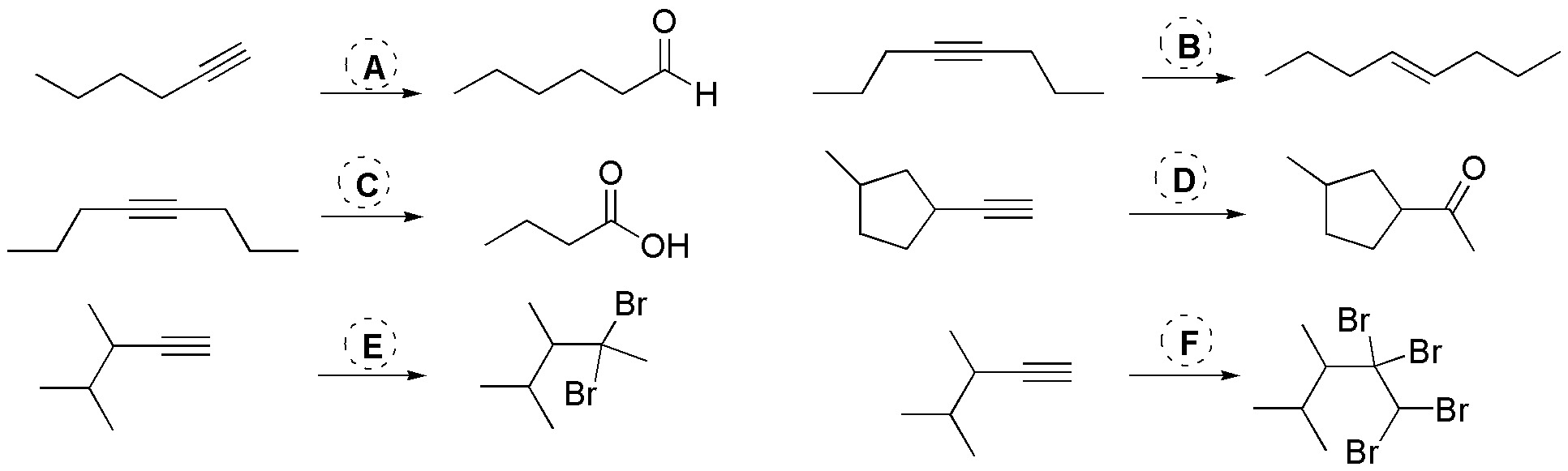
Problem 24)
Indicate the reaction conditions and reagents required to, from acetylene, obtain:
- a) pent-2-ino
- b) (E)-pent-2-ene
- c) (Z)-pent-2-ene
- d) pentane
- e) acetone
- f) propanal
Problem 25)
Devise a synthesis to obtain the following compound from ethene (acetylene).
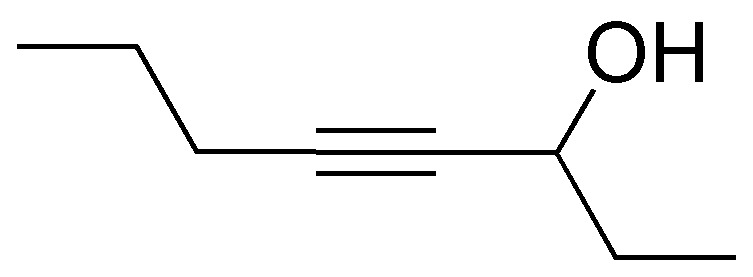
Problem 26)
What product(s) or will be obtained in the acid-catalyzed hydration and mercuric salts of the following alkynes?
- a) ethyne
- b) propyne
- c) 1-butene
- d) 2-butene
- e) 2-methylhex-3-ino
Problem 27)
Indicate what would be the products obtained from propino by treatment with the following reagents:
- a) D2 / Lindlar cat.
- b) Na / liquid ND3
- c) 1 equivalent of HI
- d) 2 equivalents of ICl
- e) 2 equivalents of HI
- f) 2 equivalents of bromine
- g) H2O / H2SO4 / HgSO4
- h) Dicyclohexylborane and subsequent treatment with H2O2 in a basic medium.