Written by J.A Dobado | Last Updated on April 22, 2024
What is recrystallization?
In a typical laboratory experiment, a solid that separates from the reaction crude is usually accompanied by impurities, so it is necessary to carry out purification (recrystallization).
By means of a technique called recrystallization, many solids can be purified using pure solvents or solvent mixtures.
Recrystallization is based on the different solubility of a solid substance in a solvent at room temperature or when the solvent is hot.
The recrystallization process is carried out with loss of product, thus affecting the overall reaction yield.
The crucial point in the crystallization process is the choice of solvent which must comply with the following properties:
- Total solubility of the substance to be purified at high temperatures.
- Low capacity to dissolve impurities that contaminate the product in any temperature range at any temperature range.
- Absence of chemical reaction with the product to be purified.
- Generation of good crystals of the product to be purified.
- Easy extraction.
For practical purposes, two types of recrystallization can be distinguished: in water and in organic solvents.
Recrystallization in water
Many organic compounds are insoluble in water at room temperature, but soluble when heated. For this purpose, a suspension of the solid in the minimum amount of water is prepared in a beaker or Erlenmeyer flask, and the mixture is brought to a boil.
If the solid does not dissolve under these conditions, small amounts are added and the water is boiled again until the compound dissolves.
It should be noted that suspended particles corresponding to part of the insoluble impurities should not be dissolved.
If the solid is observed to be dark in color, the addition of small amounts of activated carbon (activated charcoal) (before proceeding to hot filtration) will retain most of these colored impurities.
Procedure in water
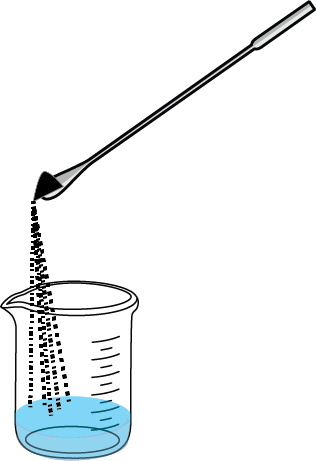
- Transfer the solid to be recrystallized to a beaker.
- Dissolve the substance in the minimum amount of hot solvent.
- If the initial crystals exhibit an intense color due to the presence of impurities, add a little activated carbon to remove them (before hot filtering), as the colored impurities are retained in the activated carbon.
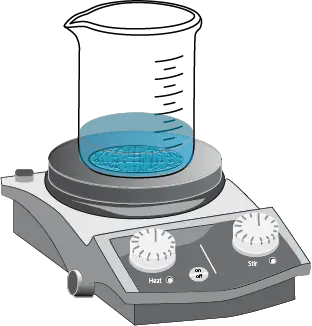
- Heat the mixture to boiling on a hot plate, verifying that the product to recrystallize has completely dissolved. Magnetic stirring (or add a piece of porous plate) should be performed to avoid sudden boiling and the formation of solid splashes.
- Disconnect the hot plate.
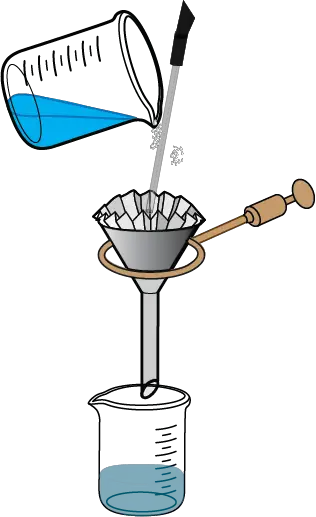

- Gravity filter the hot solution using a conical funnel and a pleated filter to remove insoluble impurities and activated carbon (discarding the solid residue, composed of activated carbon and insoluble impurities, on the filter paper). Forceps should be used to handle the beaker to avoid burns when handling hot glass vessels.
- In case the product solidifies in the funnel, add hot water to try to recover as much of it as possible.
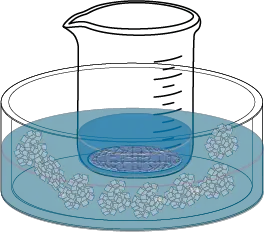
- As the solution cools, crystals of the corresponding product will form. External cooling aids this process.
- Finally, after the filtrate is completely cooled, the crystals obtained are vacuum filtered and washed (with the cold solvent used in the recrystallization) in a büchner to wash away the adhering product and then dried to remove solvent residues.
In case the crystallization is incomplete, concentrate the filtrate (by heating and evaporating half of the solvent) and repeat the process to obtain more of the desired solid product.
Recrystallization in organic solvents
In most cases, the organic compound to be purified cannot be recrystallized in water, it does not present a suitable solubility. It is necessary to find a suitable organic solvent.
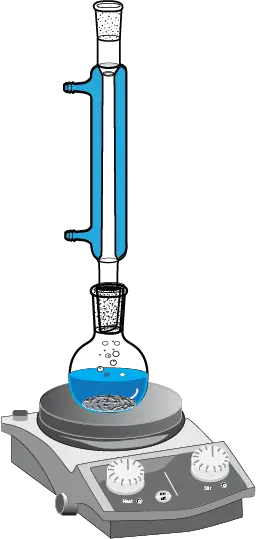
When a volatile organic solvent is used instead of water, the heating part of the solution is performed with a reflux assembly to prevent flammable volatile organic solvent vapors from causing fires.
Procedure
- A round-bottomed flask with a clamp and nut attached to a metal rack or support is placed on a hot plate.
- The solid to be recrystallized is transferred to the flask using a solid funnel.
- We add the solvent and boiling chips (or a stir bar if a magnetic stirrer is available).
- We connect a reflux condenser to the flask, connecting it to the water circuit.
- The mixture is heated to reflux to dissolve the solid. Once dissolved, turn off the hot plate and allow to cool to stop refluxing.
- While the flask is still hot, add activated carbon if necessary and gravity filter the contents of the flask with the help of beacker tongs.
- Cool the filtrate to room temperature and allow to stand until it crystallizes from the solid.
- When the filtrate is completely cooled, the crystals obtained are vacuum filtered and washed (with the cold solvent used in the recrystallization) in a büchner to wash away the adhering product and then dried to remove solvent residues.




Crystallization is sometimes facilitated by adding a few small crystals of the product or by scraping the bottom of the container with a glass rod. In both cases, crystallization nuclei are generated, which accelerate the process.
Main sources of error
- The wrong solvent is chosen for recrystallization.
- The wrong amount of solvent is used to dissolve the recrystallization solid.
- No precipitation is observed when the solution is cooled.
- Formation of an oily substance instead of obtaining a solid precipitate.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2