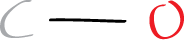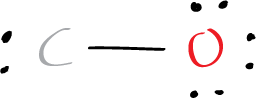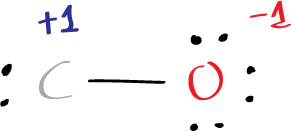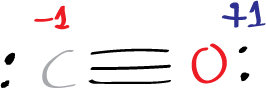Written by J.A Dobado | Last Updated on May 2, 2024
What is the Lewis structure of carbon monoxide CO?
Lewis structures are diagrams that show ho w atoms in a molecule are arranged and bonded to each other. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. We will explain step-by-step how to draw the dots of CO Lewis structure (carbon monoxide).
How to Draw the Lewis Dot Structure for Carbon monoxide
Step 1: Determine the total number of valence electrons
The first step in drawing the Lewis structure of carbon monoxide is to determine the total number of valence electrons present in the molecule. Valence electrons are the electrons in the outermost energy level of an atom that participate in chemical bonding. To determine the total number of valence electrons in carbon monoxide, we add up the valence electrons of each atom in the molecule.
Carbon (C) has 4 valence electron, and oxygen (O) has 6 valence electrons. Since there are one carbon and one oxygen atom in carbon monoxide, the total number of valence electrons is:
1×(4) + 1×(6) = 10
Step 2: Determine the central atom
There is no central atom because the molecule is diatomic.
Step 3: Connect the atoms with single bonds
Next, we need to connect the atoms with single bonds. Since carbon monoxide only has two atoms, both atoms are connected to each other with one bond (2 electrons). This gives us the following structure:
Step 4: Place the remaining electrons on the atoms
After connecting the atoms with single bonds, we need to place the remaining electrons (10 – 2 = 8) on the atoms to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons (or two electrons for hydrogen). In the case of carbon monoxide, the oxygen atom needs six more electrons to achieve a full octet, so, 3 electron pairs are added. The remaining 2 electrons (1 lone pair) are drawn as a lone pair on carbon.
To satisfy the octet rule for the oxygen atom, we place three lone pairs of electrons around the oxygen atom. This gives the oxygen atom a total of eight valence electrons (6 electrons of the lone pairs and 2 electrons of the a single O-C bond). And with respect to carbon a lone pair is added. Resulting in the following structure.
Step 5: Check the octet rule (formal charges)
The formal charge is a measure of the distribution of electrons in a molecule. It is calculated by subtracting the number of non-bonding electrons and half of the bonding electrons from the number of valence electrons in an atom. The formal charge of an atom should be as close to zero as possible. To check the formal charges in carbon monoxide, we use the following formula:
Formal charge = Valence electrons – Non-bonding electrons – 1/2 (Bonding electrons)
For the oxygen atom, the formal charge is:
6 – 6 – 1/2(2) = -1
For the carbon atom, the formal charge is:
4 – 2 – 1/2(2) = +1
Since all the formal charges in carbon monoxide are zero, this confirms that our Lewis structure is correct with respect to the total charge.
However the octet rule is wrong with respect to carbon atom with only 4 electrons from a lone pair and a bond.
For the oxygen atom, the formal charge is:
6 – 4 – 1/2(4) = 0
For the carbon atom, the formal charge is:
4 – 2 – 1/2(4) = 0
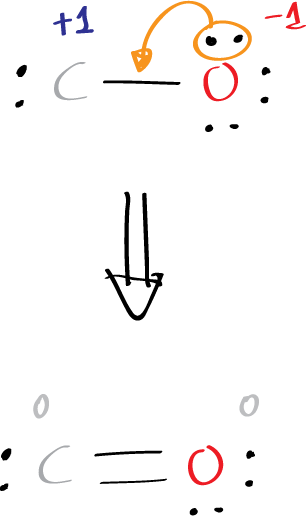
We have to correct the partial charges in both atoms and the octet rule counting for carbon. Thus, moving an electron pair from oxygen atom to form a double bond gives the resulting structure:
Now this new Lewis structure results in zero total and partial charges. However, the octet rule is not followed by carbon (2 electrons of lone pairs + 4 electrons of two bonds = 6). This is fixed if we move again another electron pair from oxygen as follows:
Finally, the octet rule is correct for carbon and oxygen and the total charge is zero.
Step 6: Draw the final structure
The final step is to draw the Lewis structure of carbon monoxide with all the lone pairs and bonds included: