Written by J.A Dobado | Last Updated on April 22, 2024
What is Chirality?
Two molecules are chiral when they are not superimposable to their mirror image. Moreover, chirality is essential for two molecules to be enantiomers. Two achiral molecules cannot be enantiomers.
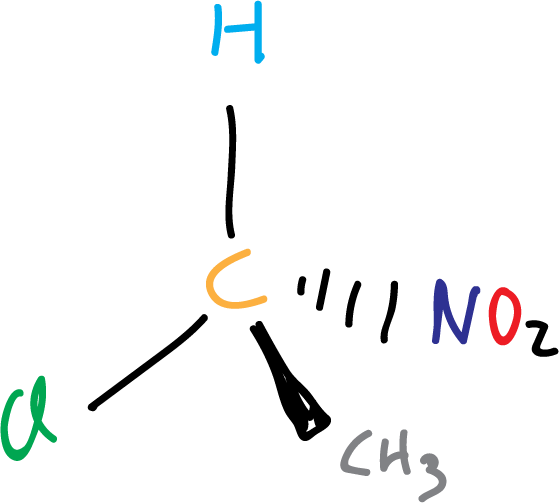
Lord Kelvin (William Thomson) in 1893, defined chirality as:
“I call chiral and say that any geometric figure, or any group of points, has chirality if its image in a plane mirror, ideally realized, cannot be made to coincide with itself.”
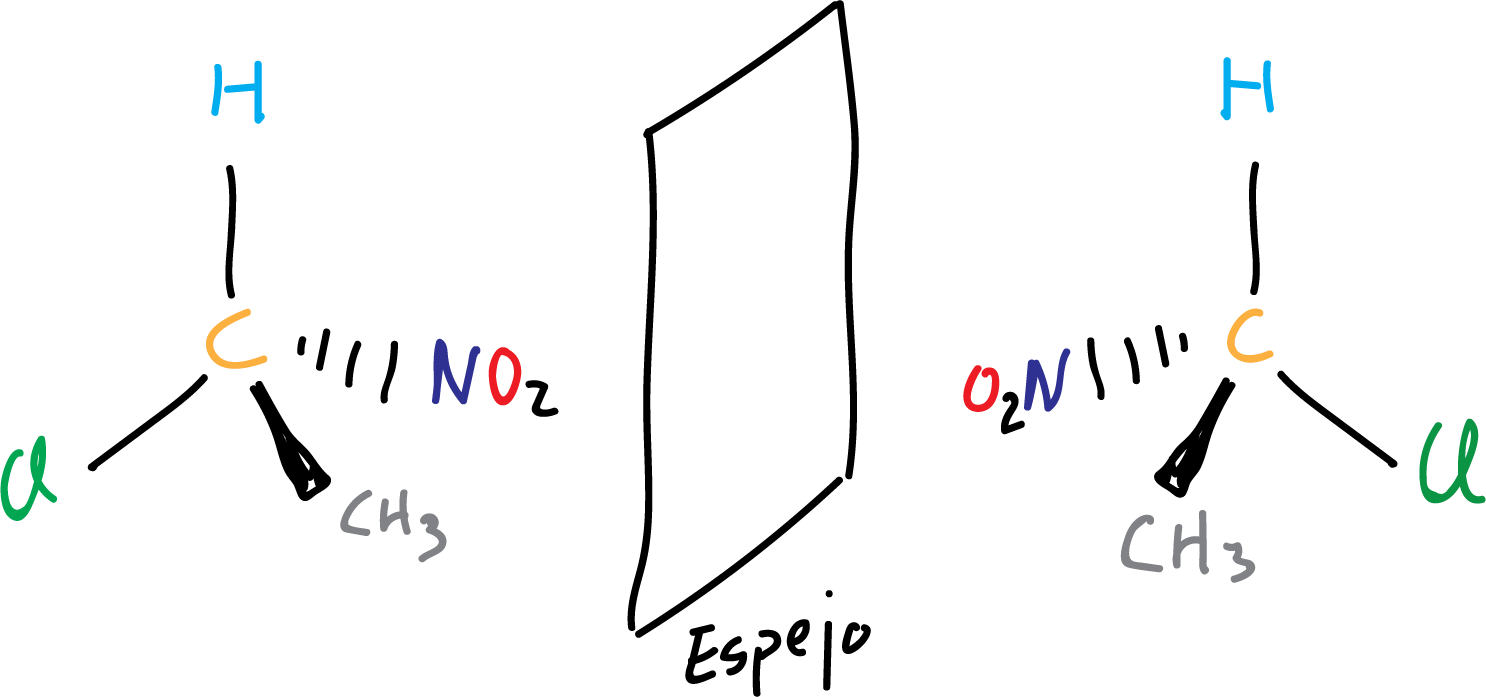
In this way to know if two molecules are chiral or not, we make a representation of the molecule and another one of its mirror image and if both cannot be superimposed (they are not identical) it means that they are chiral.
It is often used as a simile of chirality the hands, both hands are not exactly the same because they cannot be superimposed, and therefore would be chiral. In fact, the word chirality comes from the Greek word “cheir” (hand), referring to that pair of hands that are not superimposable.
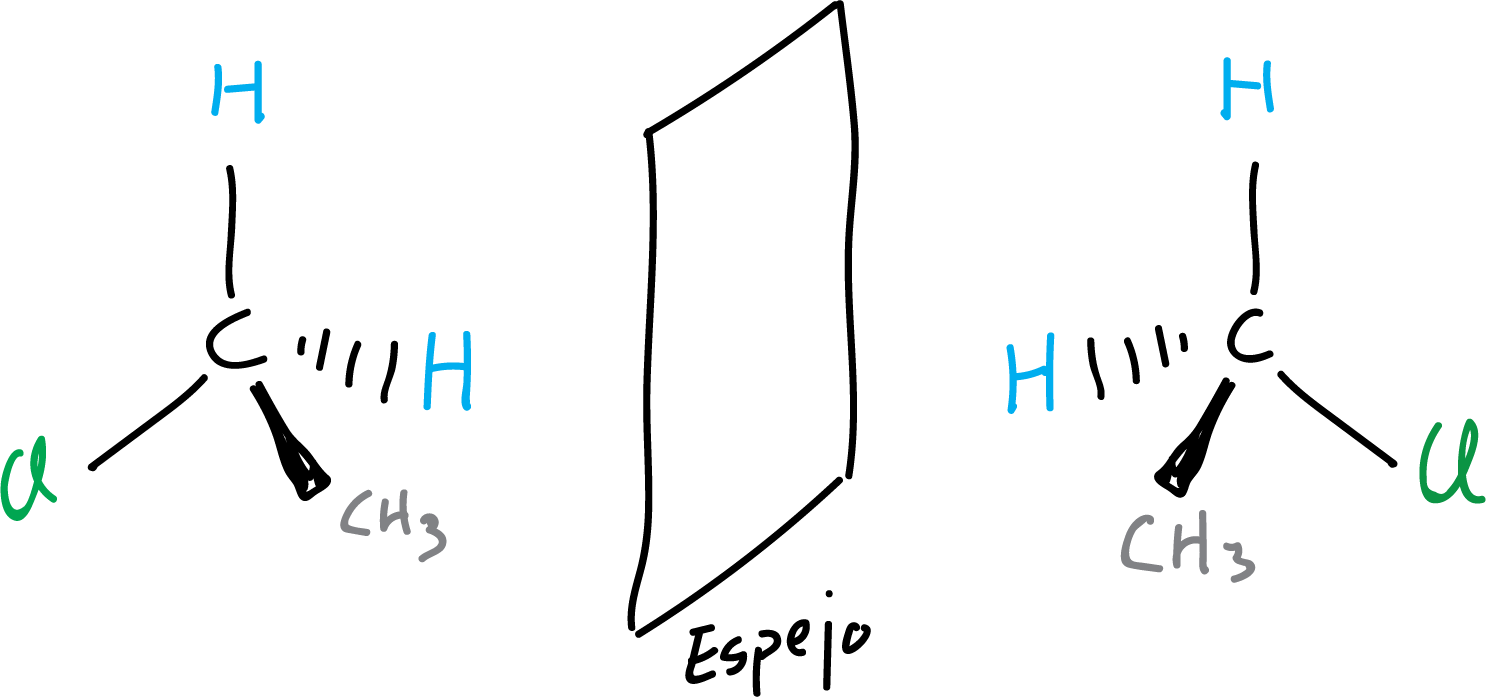
In the following example, an achiral molecule is illustrated, since both representations of the molecule and its mirror image can be superimposed (they coincide spatially when superimposed).
Chiral center
Is called chiral center a carbon bonded to four different groups. It is also called a chiral carbon, when it is to be distinguished from a chiral nitrogen or chiral phosphorus.
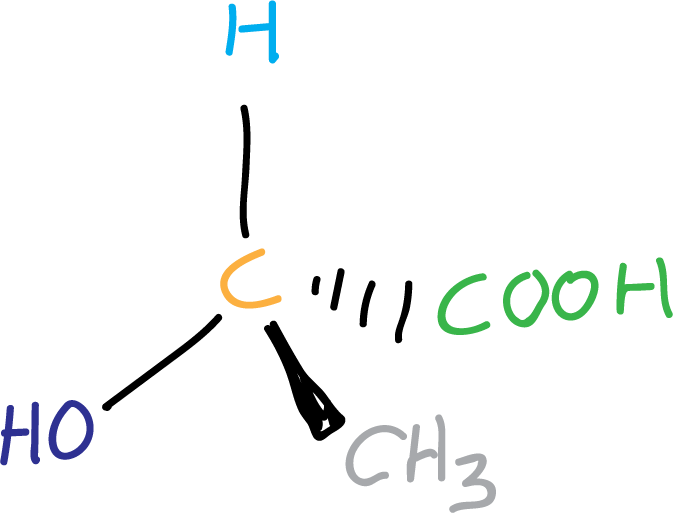
Almost all molecules with chiral centers are chiral (but not all), and almost all chiral molecules have chiral centers (but not all).
Examples
To explain this let’s look at some examples, the figure illustrates a molecule that although it has two chiral centers (highlighted in orange) is achiral.
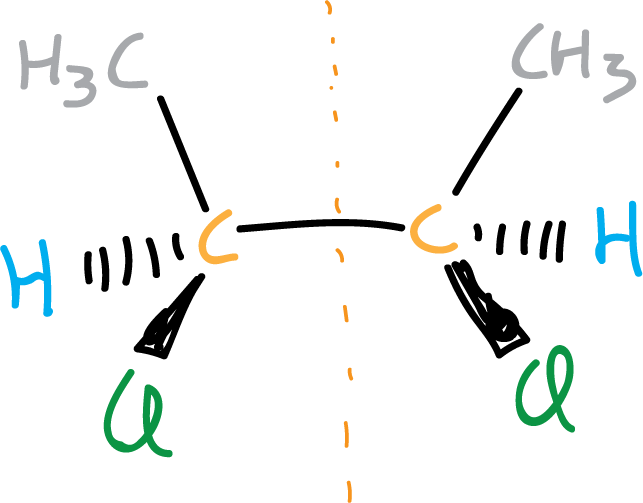
However, if there is only one chiral center in a molecule, we can say that the molecule is chiral.
fig-1 molecula con un centro quiral.
On the other hand, there are cases in which a molecule has no chiral centers and is chiral.
fig-2 sin centros quirales bisfenilo susbstituido
Chiral centers and enantiomers
Therefore, the presence or not of chiral centers is not a criterion of chirality in a molecule. However, most chiral molecules have such centers, so it is very important to know how to recognize these chiral centers. Thus, if we detect a chiral center it is very likely that the molecule is chiral and that two enantiomeric forms of the molecule can occur.
For example, limonene has an asymmetric carbon as a stereocenter. Consequently, there are two optical isomers: the enantiomer R-limonene and S-limonene. They are also called D-limonene and D-limonene (formerly called dextro and levo, respectively).
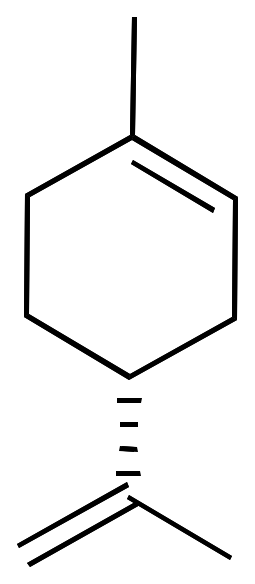 | 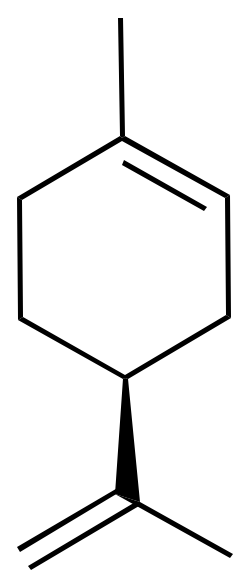 |
| 3D Structure | 3D Structure |
R-limonene is obtained commercially from lemon and exhibits a chiral rotation, [α]D, of 87–102°. R-limonene is obtained commercially from lemon and exhibits a chiral rotation, [α]D, of 87–102°.
Different types of molecular representations can be used to identify chiral centers and enantiomers.
What are enantiomers?
Isomers that are mirror images are called enantiomers. The word enantiomer comes from the Greek word meaning “opposite“.
The interesting thing about enantiomers is that they have the same physical properties (boiling point, melting point, refractive index, density, etc.), except that they bend polarized light in a different direction of rotation.
That is, the direction of rotation of the polarized light is deflected to the right by one of the enantiomers and to the left by the other, with the same absolute value but opposite sign.
On the other hand, two enantiomers also exhibit identical chemical properties (acidity constants, basicity, reactivity, reaction rate, etc.), except when optically active reagents are used.
For more information see enantiomer.