Written by J.A Dobado | Last Updated on April 22, 2024
The following is a description of the most commonly used laboratory techniques (heating and cooling) to carry out chemical reactions under different conditions.
Temperature measurement (thermometers)
In many laboratory processes, temperature measurement is required, e.g. in both hot and cold reactions. There are different types of thermometers adapted to each need and with different ranges of use. The most common ones consist of a graduated glass tube with a liquid inside. Due to the harmful nature of mercury, it has now been replaced by a colored liquid.
In some cases the thermometers are provided with a ground piece to fit the distillation heads, otherwise they will be fitted to the distillation head by means of a suitable adapter (usually a screw cap with a rubber ring).
Care must be taken to ensure that the liquid in the thermometers does not show discontinuities. Discontinuities usually occur when the thermometer reaches temperatures above its working limit. It must be ensured that the temperature range to be measured is always within the scale of the thermometer. These thermometer models are not for clinical use and should not be shaken to lower the temperature.
Nowadays, it is increasingly common to use electronic temperature measuring devices or thermocouples. These can be connected to magnetic stirrers, allowing the temperature of the plate to be regulated more reliably than the controller itself.
Heating techniques
The vast majority of organic substances are flammable and many of them are volatile, so heating a chemical reaction or dissolution poses a risk if proper precautions are not taken.
For this reason, heating techniques that involve direct exposure to a flame (due to the danger of handling volatile or flammable organic solvents), such as the typical Bunsen burners, are practically not used today. They have now been replaced by electrical devices with thermostatic heating elements.
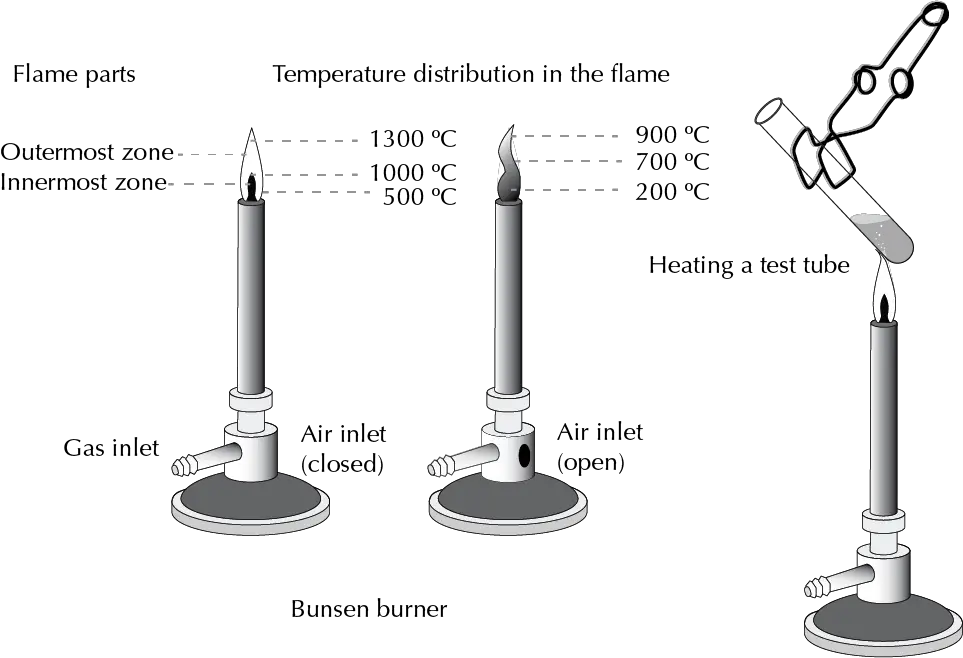
Bunsen burner
Invented in 1854 by Robert Bunsen, it is used to heat test tubes or sterilize different types of samples or instruments. In organic chemistry it is also used to mold glass rods and in the manufacture of capillaries.
A flame is generated at the end of a metal tube by a continuous stream of a gas (butane, propane or similar) which is mixed with air entering through an orifice. The air inlet can be regulated with a key or by manipulating a slit, thus modifying the composition of the gas/air mixture and the type of flame generated.
The color of the flame indicates the temperature it can reach. A yellow color (dirty flame) indicates a low temperature of approx. 300 ºC. As the air/gas ratio increases, the flame turns blue, until it becomes practically invisible, reaching the highest temperatures (approx. 700 ºC).
Although they are discouraged due to their hazardous nature, they are still used, for example, for heating test tubes in analytical tests.
The test tubes are held with a clamp, and approached to the flame slightly inclined (45°) and facing in a safe direction, with occasional shaking. So that in the event that splashing could occur, damage is minimized.
Hot plate
The most commonly used method for heating a reaction is the use of a hot plate with an internal temperature regulator. Certain plate models allow the connection of an external thermocouple for more precise temperature regulation.
The vast majority of today’s plates also include magnetic stirring, which allows simultaneous heating and stirring.
The container or assembly used is placed directly on the heat source of the plate, or a bath can be inserted, which can be water (bain-marie), oil, sand or an aluminum block. In all the cases the temperature must be controlled with a thermometer that is introduced in the bath or aluminum block.
| DANGER! “Never heat an open container with a flammable solvent on a hot plate. Vapors may cause a fire if they come in contact with the hot zone or the electrical components of the hotplate.” |
Water bath (bain-marie or double boiler)
The simplest bath still in use is the water bath or “bain-marie”, which is very effective when temperatures below 80 °C are required. With it, temperatures between 70 and 80 ºC are reached inside the reaction. The water is introduced into a crystallizer and the reaction vessel is placed inside the bath. The term “bain-marie” comes from Mary the Jewess, sister of Moses, who was considered the first alchemist.
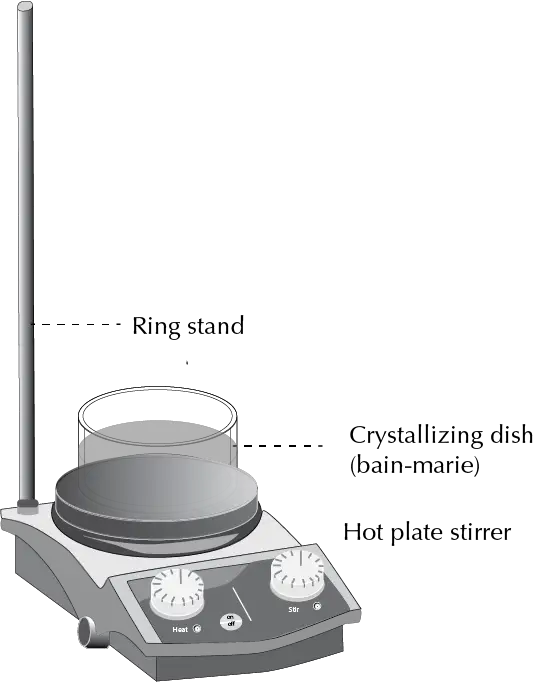
It presents the problem of evaporation relatively quickly. Rapid evaporation is prevented by adapting a piece of aluminum foil to cover the free surface of the water bath.
Oil bath
Fluids transmit heat efficiently and homogeneously and have therefore been commonly used for heating reactions. These fluids must be thermally stable and non-flammable in the temperature range to be used. Another important fact is to know the flash point. The minimum temperature at which a flammable mixture consisting of fluid vapors and air is produced in the vicinity of the fluid surface.
The fluid is introduced into a vessel, usually a glass crystallizer, which is placed over a heat source (the heating plate itself). The crystallizer has the advantage of not having a very high wall, which allows the reaction to be visualized. Mineral or silicone oils are used as fluids.
| DANGER! “Mineral oil is flammable. Special care must be taken to ensure that no drops are spilled on the hot plate. In addition, if water mixes with the oil, there is a risk of splashing if the temperature is higher than 100 ºC. The danger lies in the fact that since the water is denser than the oil, it falls to the bottom and at that temperature it will produce splashes with the projection of oil at high temperature.” |
Sand bath
Instead of a fluid, a crystallizer can be filled with sand. It allows heating small vessels to temperatures not exceeding 200 ºC. They are prepared by adding sand to the crystallizer to a height of about 3 cm.
Aluminum block
It is an accessory that is placed on the heating plate, producing an efficient heat transfer between the heating plate and the reaction vessel.
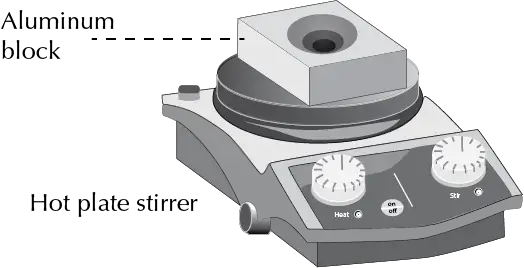
There are several designs, ranging from hemispherical flasks that adapt to round bottom flasks, improving their stability, to others with different holes in the block for different types of containers (multi-well holder).
In this case, it is possible to heat several flasks or vials at the same time. Aluminum blocks have a number of advantages:
- Aluminum heats up very quickly.
- It can reach high temperatures.
- The blocks can be cooled quickly if they are picked up with tongs and immersed in water.
Table 1 lists some of the materials used for the baths, as well as the commonly used temperature range and flash point.
| Material | Flash point (ºC) | Operating temperature (ºC) | Comments |
| Water | – | 30-70 | Inexpensive; non-flammable and non-toxic; no residues; evaporates quickly |
| Mineral oil | 113 | 30-90 | Inexpensive; darkens and polymerizes over time; hazardousa; low flash point |
| Paraffin wax | –b | 55-180 | Inexpensive; does not degrade over time; dangerousa |
| Silicone oil | 150-350 | 30-230 | Wide temperature range; relatively stable with use; hazardousa |
| Sand | – | 30-500 | Inexpensive; non-flammable; for practical purposes, no limit of use |
| aMay produce projections if water is accidentally spilled. bVariable depending on the origin. | |||
| DANGER! “Never heat a bath of a fluid to a temperature higher than 20 ºC below its flash point. |
Thermostatic bath
They are autonomous equipment consisting of a container for water or oil that is heated by means of a resistance, being able to maintain a stable temperature for long periods of time.
Heating mantle
It has a metal casing with a cavity in which there is a bed of fiberglass or ceramic material that adapts to the shape of the vessel, which can be kept in direct contact with the glass of the vessel. It has an electrical resistance that allows temperature control by means of a power regulator connected to a thermostat.
Heating mantles with magnetic stirring system are available, so that heating and stirring can be carried out at the same time. The most common are those specially designed for heating round-bottomed flasks from 50 ml to 5 l and even larger.
The use of this heating system is very convenient for flasks with capacities above 500 ml, especially for the distillation of solvents in large quantities and where the required temperatures are relatively high and somewhat stable. Heating plates are more commonly used for smaller flasks.
The quickest way to change the heating rate of a heating mantle is to change the distance between the cavity and the flask, rather than manipulating the thermostat. This separation can be changed by raising and lowering the flask by means of a suitable support mounted outside the heating mantle that allows this to be done quickly.
Although heating blankets are very easy to use and safe to use, extreme caution must be exercised and any chemical products, including solvents, must be avoided at all costs, for two reasons:
- The temperature reached by the resistor is such that it causes thermal decomposition of the substances, generating toxic vapors or fire hazard.
- The device deteriorates and can cause malfunctions and even short circuits.
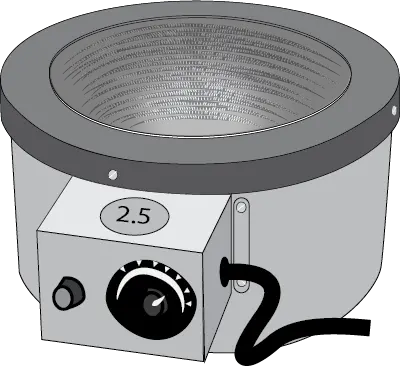
Less common in an organic chemistry laboratory are heating mantles designed for heating beakers, which, unlike the previous ones, are cylindrical instead of hemispherical in shape.
Microwave equipment
An alternative to the use of the aforementioned devices is microwave equipment. These can provide thermal energy to a reaction with the added advantage of saving time and energy.
This form of heating utilizes the property of some molecules to transform electromagnetic energy (near 900 MHz) into heat, which causes the rotation of dipoles within the liquid causing polar molecules to align and then relax in the oscillating field of electromagnetic radiation.
When the energy of the rotation of these dipoles is dissipated, heating of the liquid occurs. Because of this, as the heating occurs inside the liquid and is not transferred from the vessel to the liquid, the liquid will be at a higher temperature than the vessel.
However, they have the disadvantage that in order to adequately reproduce the results, sophisticated equipment is needed, with prices that are not usually affordable to most practice laboratories.
Melting furnace
They are mainly used for metal smelting and synthesis of ceramic materials. They are also used to activate molecular sieves and to obtain anhydrous salts.
Cooling techniques
Sometimes it is necessary to cool a reaction below room temperature, for example, in very exothermic reactions where it is necessary to remove or mitigate the heat given off, or because the reactants or products are thermally unstable.
In other cases, it is necessary to cool a solution simply to promote the crystallization of a certain compound or even to facilitate the condensation of a liquid. Some procedures used in the laboratory for cooling are described below.
Ice bath
This is the simplest option. A crystallizer is filled with ice, which must be crushed and mixed with water to facilitate contact with the flask to be cooled.
Cryogenic agent
Cryogenic agens are usually binary mixtures that acquire a lower temperature than that of the separate components. The simplest of all is the mixture composed of ice and common salt.
In this case, it is somewhat difficult to fix the temperature, since it depends on the ratio between the quantities of ice and salt. Table 2 lists the different cryogenic agents classified by temperature.
| Temperature (ºC) | Compositiona,b |
| 13 | p-Xylene/CO2(s) |
| 12 | Dioxane/CO2(s) |
| 6 | Cyclohexane/CO2(s) |
| 5 | Benzene/CO2(s) |
| 2 | Formamide/CO2(s) |
| 0 | Ice |
| -5 a -20 | Ice/NaCl |
| -10.5 | Ethylen glycol/CO2(s) |
| -12 | Cycloheptane/CO2(s) |
| -15 | Benzyl alcohol/CO2(s) |
| -22 | Tetrachloroethylene/CO2(s) |
| -22.8 | Carbon tetrachloride/CO2(s) |
| -25 | 1,3-Dichlorobenzene/CO2(s) |
| -29 | or-Xylene/CO2(s) |
| -32 | m-Toluidine/CO2(s) |
| -38 | Heptan-3-one/CO2(s) |
| -41 | Acetonitrilo/CO2(s) |
| -42 | Pyridine/CO2(s) |
| -47 | m-Xylene/CO2(s) |
| -56 | n-Octane/CO2(s) |
| -60 | Isopropyl ether/CO2(s) |
| -61 | Chloroform/CO2(s) |
| -72 | EtOH/CO2(s) |
| -77 | Butyl acetate/CO2(s) |
| -78 | Acetone/CO2(s) |
| -83 | Propyl amine/CO2(s) |
| -83.6 | Ethyl acetate/N2(l) |
| -89 | n-Butanol/N2(l) |
| -94 | Hexane/N2(l) |
| -94.6 | Acetone/N2(l) |
| -95.1 | Toluene/N2(l) |
| -98 | MeOH/N2(l) |
| -100 | Diethyl ether/CO2(s) |
| -104 | Cyclohexane/N2(l) |
| -116 | EtOH/N2(l) |
| -116 | Diethyl ether/N2(l) |
| -131 | n-Pentane/N2(l) |
| -160 | Isopentane/N2(l) |
| -196 | N2(l) |
| aCO2(s) = dry ice. bN2(l) = liquid nitrogen. | |
If the reaction temperature value is not critical, the baths can be replaced by others that give a similar temperature and the solvents used are less toxic, or cheaper.
For the reactions that are usually carried out in a practice laboratory, baths with temperatures above 0 ºC can be replaced by ice/water mixtures by controlling the temperature with a thermometer. For temperatures between 0 and -20 ºC the ideal solution due to its low price and availability is to use ice or ice/salt mixtures.
In case it is necessary to maintain the temperature for long periods of time, it is convenient to isolate the vessel in which the cryogenic agent is kept and the flask in which the reaction is carried out.
Cryogenic agents, especially those involving the use of more or less toxic or hazardous solvents, are being replaced by chillers.
Thermostatic cooling bath
The ideal procedure to maintain a reaction at low temperature is to use a thermostatic cooling bath, generally consisting of a metal basin filled with a liquid that does not freeze at the working temperature, in which the reaction vessel is immersed.
Immersion chiller
They have a refrigeration unit to produce cold, but with an external accessory (cooling coil, similar to a cold finger) that can be used to generate localized cold in other types of reaction vessels.
Closed-loop chiller systems
The so-called closed circuit coolers are widely used to circulate liquids at low temperature in the usual water condensers. Their most common use is in rotary evaporator and reflux of low-boiling solvents, they are usually filled with a refrigerant mixture similar to the antifreeze used in the automotive industry.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2