Written by J.A Dobado | Last Updated on May 2, 2024
What is the Electron Configuration?
The electron configuration is the summary of where the electrons are located around a nucleus. Each neutral atom has a number of electrons equal to its number of protons. Therefore, these electrons are located in orbitals in an arrangement around the nucleus. The notation indicates that indicates their energy and the type of orbital in which they are located. The types of orbitals and how many electrons each can accommodate is summarized as follows up to the n=4 level.
| Level (n) | sub-level | No. orbitals for each type | No. orbitals for each level | No max. e– (2n2) |
| 1 | s | 1 | 1 | 2 |
| 2 | s | 1 | 4 | 8 |
| p | 3 | |||
| 3 | s | 1 | 9 | 18 |
| p | 3 | |||
| d | 5 | |||
| 4 | s | 1 | 16 | 32 |
| p | 3 | |||
| d | 5 | |||
| f | 7 |
Then, according to the above table, it needs 2 electrons to fill an orbital s, 6 electrons to fill an orbital p, 10 electrons to fill an orbital d and 14 electrons to fill the orbital f.
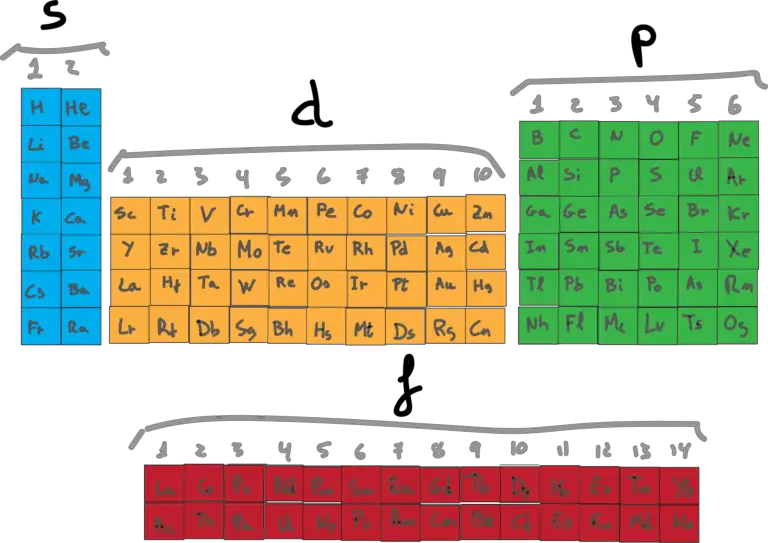
However, in order to determine the electron configuration of every element in the periodic table, we will need to know the order of filling of these orbitals.
Order of filling
The order in which the electrons are placed in orbitals is based on the energy of these. Thus gradually filling up by order of this energy according to the Aufbau principle.
The Aufbau principle derived from the Schrödinger equation, which describes the behavior of electrons in an atom.
According to the Aufbau principle, electrons fill the lowest energy orbitals first before occupying higher energy orbitals. This means that in a ground state atom, the electrons will always occupy the orbitals with the lowest energy levels first, and then fill higher energy levels as needed.
The orbitals of lowest energy are filled first, and is determined in the following way:
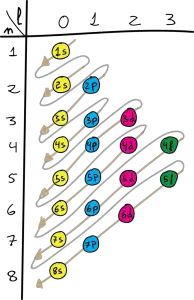
1s2→2s2→2p6→3s2→3p6→4s2→3d10→4p6→5s2→4d10→5p6→6s2→4f 14→5d10→6p6→7s2→5f 14→6d10→7p6 (6f 14 7d10 7f 14) → 8s2 …
The order of filling of the last three layers are described, has not been able to determine. This is mainly due to non-availability of the necessary amount of the corresponding items to make experimental measurements of physico-chemical properties.
Keep in mind that this order is an estimate, and for certain elements varies as there are configurations that are more stable when the orbitals are filled, semillenos, or empty.
To write the electron configuration, we start with the layer number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example, an atom of carbon has 6 electrons would be:
Carbon: 1s2 2s2 2p4
Often abbreviated notation, using the noble gas preceding in order of atomic number in square brackets. For the carbon would be:
Carbon: [He] 2s22p4
The order of filling orbitals follows a specific pattern, which is based on the periodic table. The first two electrons occupy the 1s orbital, followed by two electrons in the 2s orbital. The 2p orbitals are then filled with six electrons, before the 3s orbital is filled with two electrons. This pattern continues until all of the electrons are accounted for.
The Aufbau principle is a useful tool for predicting the electron configurations of atoms and molecules. By following the order of filling orbitals based on the principle, we can determine the electron configuration of an atom or ion, which is essential for understanding its chemical and physical properties.
However, it is important to note that the Aufbau principle is a simplified model that does not account for some exceptions and anomalies that occur in certain elements. For example, the electron configurations of some transition metals and lanthanides are not strictly predictable based on the Aufbau principle alone. Nonetheless, the principle remains a useful starting point for understanding the behavior of electrons in atoms and molecules.
Electron configuration table
The following table shows the electron configuration of the elements, ordered by their atomic number (Z).
| Z | Symbol | Configuration |
|---|---|---|
| 1 | H | 1s1 |
| 2 | He | 1s2 |
| 3 | Li | [He] 2s1 |
| 4 | Be | [He] 2s2 |
| 5 | B | [He] 2s2 2p1 |
| 6 | C | [He] 2s2 2p2 |
| 7 | N | [He] 2s2 2p3 |
| 8 | O | [He] 2s2 2p4 |
| 9 | F | [He] 2s2 2p5 |
| 10 | Ne | [He] 2s2 2p6 |
| 11 | Na | [Ne] 3s1 |
| 12 | Mg | [Ne] 3s2 |
| 13 | Al | [Ne] 3s2 3p1 |
| 14 | Si | [Ne] 3s2 3p2 |
| 15 | P | [Ne] 3s2 3p3 |
| 16 | S | [Ne] 3s2 3p4 |
| 17 | Cl | [Ne] 3s2 3p5 |
| 18 | Ar | [Ne] 3s2 3p6 |
| 19 | K | [Ar] 4s1 |
| 20 | Ca | [Ar] 4s2 |
| 21 | Sc | [Ar] 3d1 4s2 |
| 22 | Ti | [Ar] 3d2 4s2 |
| 23 | V | [Ar] 3d3 4s2 |
| 24 | Cr | [Ar] 3d5 4s1 |
| 25 | Mn | [Ar] 3d5 4s2 |
| 26 | Fe | [Ar] 3d6 4s2 |
| 27 | Co | [Ar] 3d7 4s2 |
| 28 | Ni | [Ar] 3d8 4s2 |
| 29 | Cu | [Ar] 3d10 4s1 |
| 30 | Zn | [Ar] 3d10 4s2 |
| 31 | Ga | [Ar] 3d10 4s2 4p1 |
| 32 | Ge | [Ar] 3d10 4s2 4p2 |
| 33 | As | [Ar] 3d10 4s2 4p3 |
| 34 | Se | [Ar] 3d10 4s2 4p4 |
| 35 | Br | [Ar] 3d10 4s2 4p5 |
| 36 | Kr | [Ar] 3d10 4s2 4p6 |
| 37 | Rb | [Kr] 5s1 |
| 38 | Sr | [Kr] 5s2 |
| 39 | Y | [Kr] 4d1 5s2 |
| 40 | Zr | [Kr] 4d2 5s2 |
| 41 | Nb | [Kr] 4d4 5s1 |
| 42 | Mo | [Kr] 4d5 5s1 |
| 43 | Tc | [Kr] 4d5 5s2 |
| 44 | Ru | [Kr] 4d7 5s1 |
| 45 | Rh | [Kr] 4d8 5s1 |
| 46 | Pd | [Kr] 4d10 |
| 47 | Ag | [Kr] 4d10 5s1 |
| 48 | Cd | [Kr] 4d10 5s2 |
| 49 | In | [Kr] 4d10 5s2 5p1 |
| 50 | Sn | [Kr] 4d10 5s2 5p2 |
| 51 | Sb | [Kr] 4d10 5s2 5p3 |
| 52 | Te | [Kr] 4d10 5s2 5p4 |
| 53 | I | [Kr] 4d10 5s2 5p5 |
| 54 | Xe | [Kr] 4d10 5s2 5p6 |
| 55 | Cs | [Xe] 6s1 |
| 56 | Ba | [Xe] 6s2 |
| 57 | La | [Xe] 5d1 6s2 |
| 58 | Ce | [Xe] 4f 1 5d1 6s2 |
| 59 | Pr | [Xe] 4f 3 6s2 |
| 60 | Nd | [Xe] 4f 4 6s2 |
| 61 | Pm | [Xe] 4f 5 6s2 |
| 62 | Sm | [Xe] 4f 6 6s2 |
| 63 | Eu | [Xe] 4f 7 6s2 |
| 64 | Gd | [Xe] 4f 7 5d1 6s2 |
| 65 | Tb | [Xe] 4f 9 6s2 |
| 66 | Dy | [Xe] 4f 10 6s2 |
| 67 | Ho | [Xe] 4f 11 6s2 |
| 68 | Er | [Xe] 4f 12 6s2 |
| 69 | Tm | [Xe] 4f 13 6s2 |
| 70 | Yb | [Xe] 4f 14 6s2 |
| 71 | Lu | [Xe] 4f 14 5d1 6s2 |
| 72 | Hf | [Xe] 4f 14 5d2 6s2 |
| 73 | Ta | [Xe] 4f 14 5d3 6s2 |
| 74 | W | [Xe] 4f 14 5d4 6s2 |
| 75 | Re | [Xe] 4f 14 5d5 6s2 |
| 76 | Os | [Xe] 4f 14 5d6 6s2 |
| 77 | Ir | [Xe] 4f 14 5d7 6s2 |
| 78 | Pt | [Xe] 4f 14 5d9 6s1 |
| 79 | Au | [Xe] 4f 14 5d10 6s1 |
| 80 | Hg | [Xe] 4f 14 5d10 6s2 |
| 81 | Tl | [Xe] 4f 14 5d10 6s2 6p1 |
| 82 | Pb | [Xe] 4f 14 5d10 6s2 6p2 |
| 83 | Bi | [Xe] 4f 14 5d10 6s2 6p3 |
| 84 | Po | [Xe] 4f 14 5d10 6s2 6p4 |
| 85 | At | [Xe] 4f 14 5d10 6s2 6p5 |
| 86 | Rn | [Xe] 4f 14 5d10 6s2 6p6 |
| 87 | Fr | [Rn] 7s1 |
| 88 | Ra | [Rn] 7s2 |
| 89 | Ac | [Rn] 6d1 7s2 |
| 90 | Th | [Rn] 6d2 7s2 |
| 91 | Pa | [Rn] 5f 2 6d1 7s2 |
| 92 | U | [Rn] 5f 3 6d1 7s2 |
| 93 | Np | [Rn] 5f 4 6d1 7s2 |
| 94 | Pu | [Rn] 5f 6 7s2 |
| 95 | Am | [Rn] 5f 7 7s2 |
| 96 | Cm | [Rn] 5f 7 6d1 s2 |
| 97 | Bk | [Rn] 5f 9 s2 |
| 98 | Cf | [Rn] 5f 10 s2 |
| 99 | Es | [Rn] 5f 11 s2 |
| 100 | Fm | [Rn] 5f 12 s2 |
| 101 | Md | [Rn] 5f 13 s2 |
| 102 | No | [Rn] 5f 14 s2 |
| 103 | Lr | [Rn] 5f 14 7s2 7p1 |
| 104 | Rf | [Rn] 5f 14 6d2 7s2 |
| 105 | Db | [Rn] 7s2 5f 14 6d3 |
| 106 | Sg | [Rn] 7s2 5f 14 6d4 |
| 107 | Bh | [Rn] 7s2 5f 14 6d5 |
| 108 | Hs | [Rn] 7s2 5f 14 6d6 |
| 109 | Mt | [Rn] 7s2 5f 14 6d7 |
| 110 | Ds | [Rn] 7s25f 14 6d8 |
| 111 | Rg | [Rn] 5f 14 6d10 7s1 |
| 112 | Cn | [Rn] 5f 14 6d10 7s2 |
| 113 | Nh | [Rn] 5f 14 6d10 7s2 7p1 |
| 114 | Fl | [Rn] 5f 14 6d10 7s2 7p2 |
| 115 | Mc | [Rn] 5f 14 6d10 7s2 7p3 |
| 116 | Lv | [Rn] 5f 14 6d10 7s2 7p4 |
| 117 | Ts | [Rn] 5f 14 6d10 7s2 7p5 |
| 118 | Og | [Rn] 5f 14 6d10 7s2 7p6 |
Electron Configuration Video
Frequently asked questions about electronic configuration
- What is the electron configuration?
The electron configuration is a description of the electronic structure of an atom. The orbitals are filled with electrons in all shells following three rules: Aufbau’s principle, Pauli’s exclusion principle and Hund’s rule.
- What is the electron configuration of the chromium atom (Cr Z = 24)?
The expected electron configuration for chromium is 1s2 2s2 2p6 3s2 3p6 3d4 4s2. However, half-filled and fully filled subshell present extra stability, and, thus, the correct electron configuration of crhomium atom [Z = 24] is [Ar] 3d5 4s1.