Go to the page with the solutions to the problems.
Organic Synthesis – list of problems
The following list of Organic Synthesis problems deals with the three aspects described below:
- Reactivity of molecules with more than one functional group.
- Synthetic sequences involving the chemistry of different functional groups.
- Preparation of molecules from fragments with a smaller number of carbon atoms.
Problem 1)
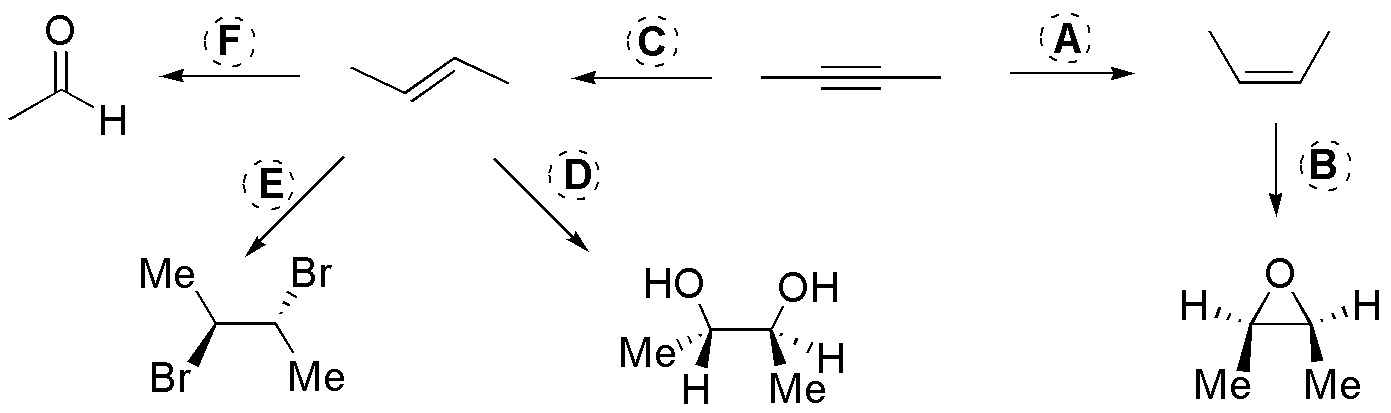
Problem 2)

Problem 3)

Problem 4)
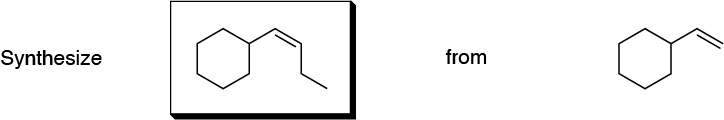
Problem 5)
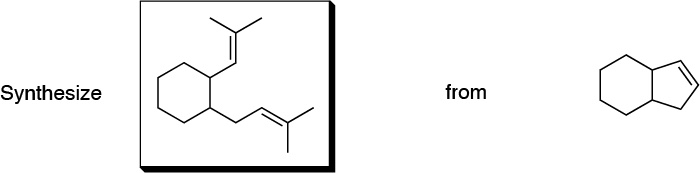
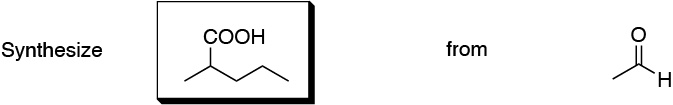
Problem 6)

Problem 7)

Problem 8)

Problem 9)
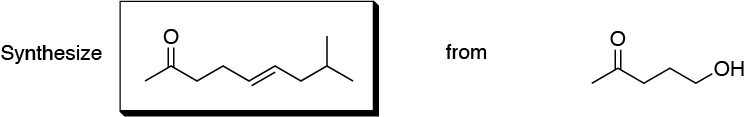
Problem 10)

Problem 11)
From acetylene and as many reagents as you think necessary, prepare the Z and E isomers of 6-methylhept-3-ene.
Problem 12)
Obtain 1-phenylpropan-2-one from (bromomethyl)benzene.
Problem 13)
Design a procedure to obtain 2-phenylacetamide from bromobenzene.
Problem 14)
Suggest a procedure to perform the following transformations:
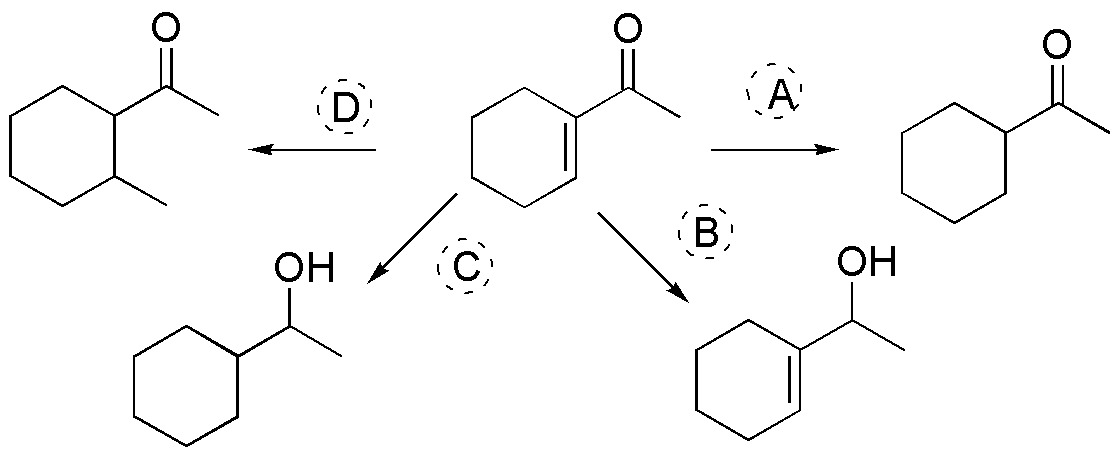
Problem 15)
Pentyl bromide is reacted with magnesium in ether to give compound A. A reacts with ethylene oxide followed by aqueous acid to give B. When B is oxidized with PPC in CH2Cl2 it gives C, which reacts with phenylmagnesium bromide to give D, after treating with aqueous hydrochloric acid. Determine the structures of compounds A, B, C and D, based on the sequence of reactions described.
Problem 16)
Describe a procedure to obtain 6-methyltetrahydro-2H-pyran-2-one (δ-caprolactone) from 4-bromobutanal.
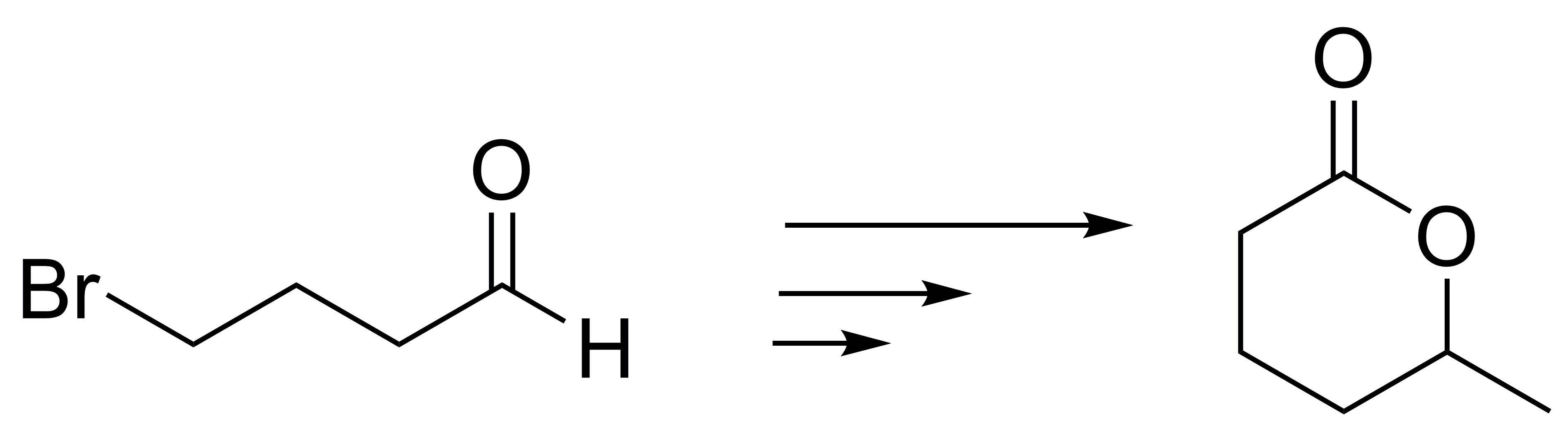
Problem 17)
Employing cyclohex-2-enone as starting material prepare 4-oxocyclohex-2-enocarboxylic acid.
Problem 18)
Ethyl p-aminobenzoate is commercially known as benzocaine. It is widely used in medicine as a local anesthetic. Design a procedure to obtain such a product from benzene.
Problem 19)
Design a procedure for the synthesis of methyl pentanoate from 1-bromobutane.
Problem 20)
Using as starting products or carbon source compounds (I-V), design a synthetic procedure for the preparation of a) and b).
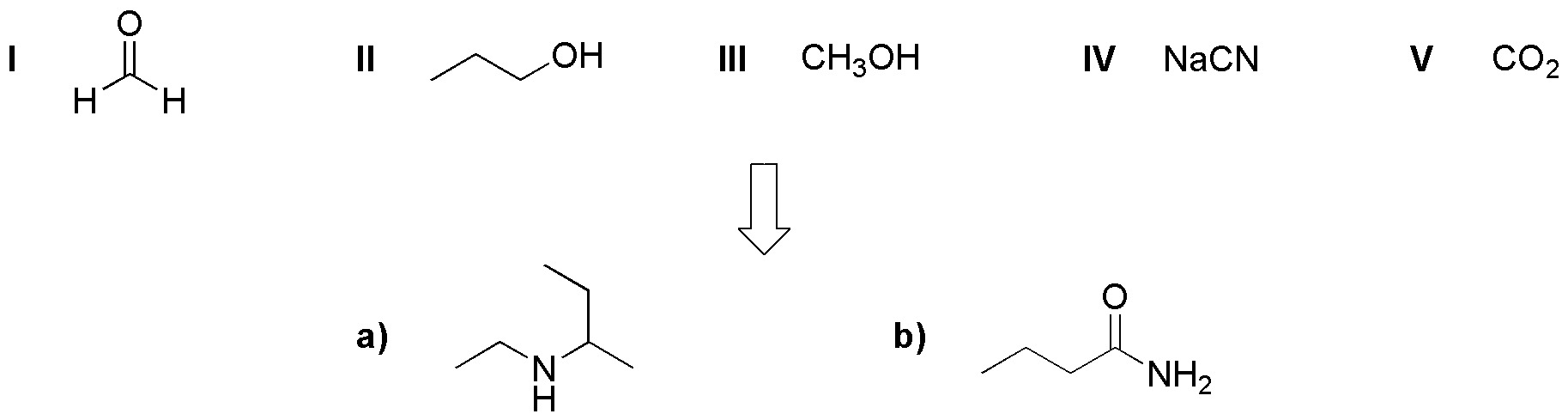
Problem 21)
Starting from ethanol, as the only carbon compound, describe a synthesis for the following molecules:
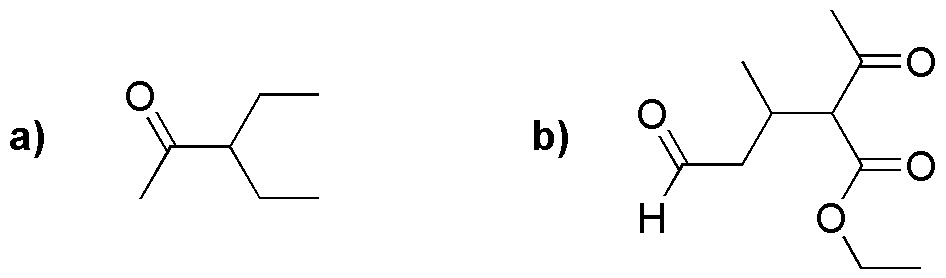
Problem 22)
Deduce in a reasoned way, which compounds from the following list will be obtained more easily by a malonic synthesis.

Problem 23)
An alkene of molecular formula C6H12 gives by ozonolysis propanal as the only product. Treatment with a peracid leads to an optically active compound. Propose the structure of such an alkene.
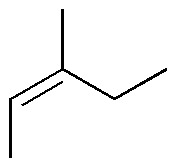
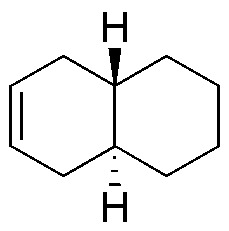
Problem 24)
Complete the following table, taking into account the stereochemistry of the compounds obtained (A-X).
| Br2 / Cl4C | H2O2 / OsO4 (cat.) | Ozonolysis | KMnO4 (hot) | Peracids | HBr / peroxides | |
|---|---|---|---|---|---|---|
 | A | B | C | D | E | F |
| G | H | I | J | K | L | |
 | M | N | O | P | Q | R |
| S | T | U | V | W | X |
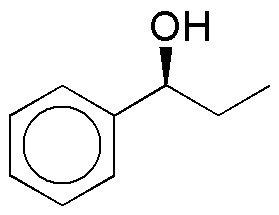
Problem 25)
Complete the following table (A-I) of reactivity of alcohols.
| PCC /CH2Cl2 | CrO3 / H3O+ | KMnO4 | |
|---|---|---|---|
 | A | B | C |
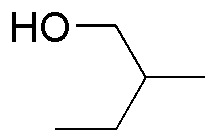 | D | E | F |
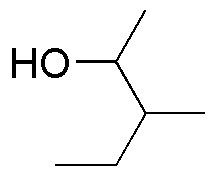 | G | H | I |
Problem 26)
Complete the following table (A-P):
| LiAlH4 | NaBH4 | B2H6 | DIBAH | |
|---|---|---|---|---|
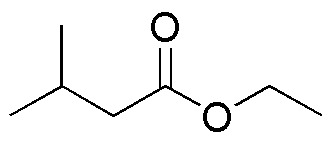 | A | B | C | D |
| E | F | G | H | |
| I | J | K | L | |
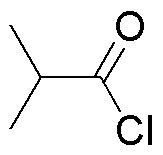 | M | N | O | P |
Problem 27)
Complete the following scheme (A-K):
Design a procedure to prepare propane-1,1,1,2,3-tetracarboxylic acid from:
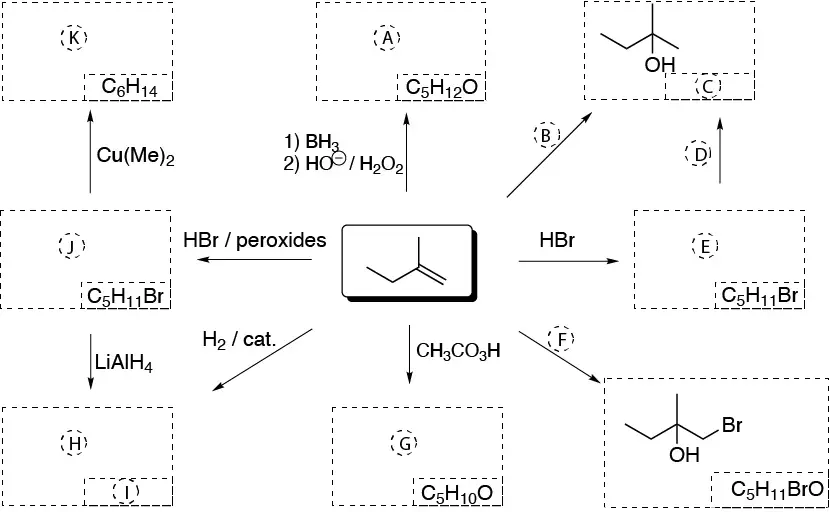
Problem 28)
Indicate the reagents (A-M) needed to perform the following transformations:
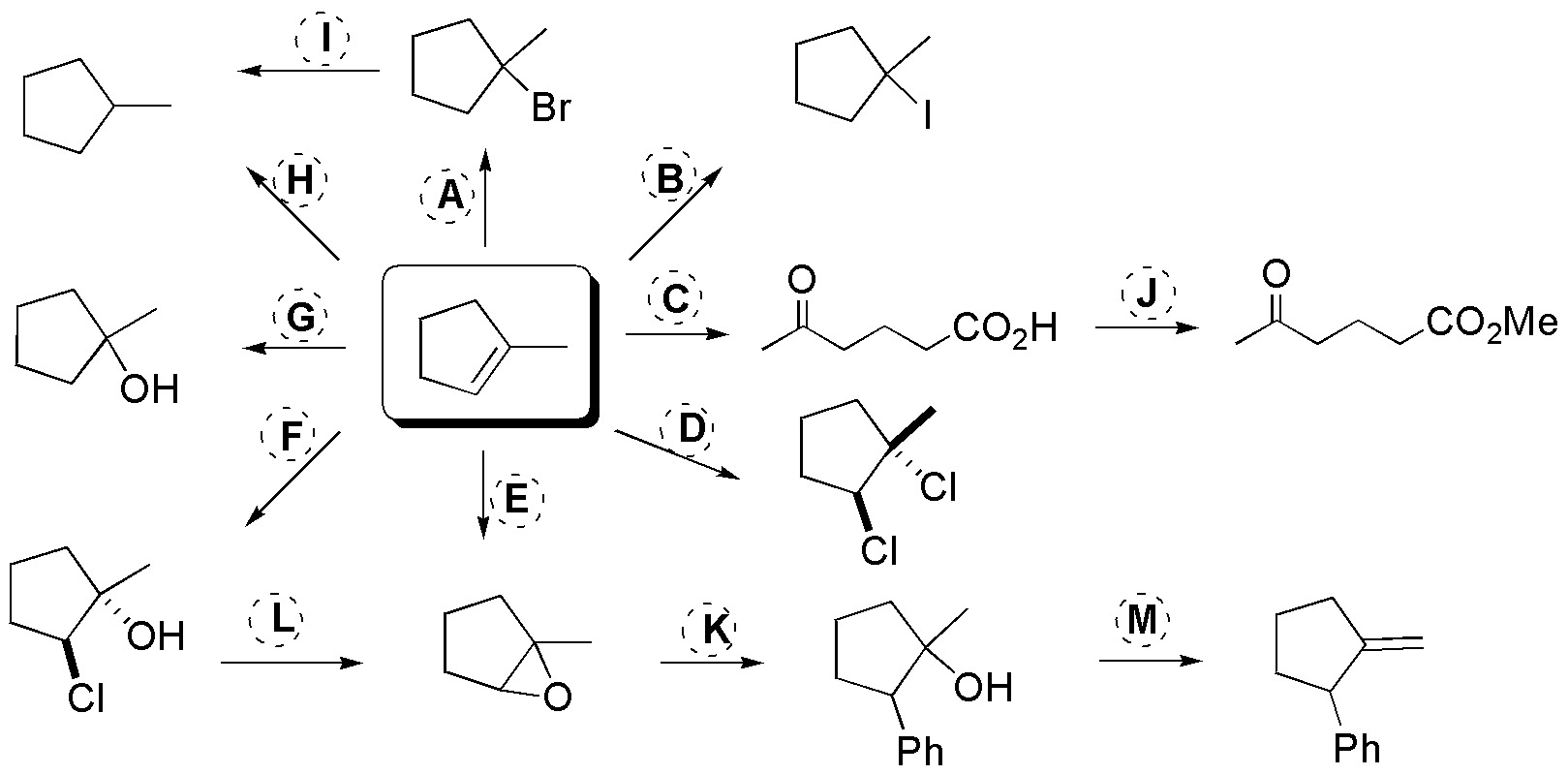
Problem 29)
Deduce the structures of the compounds A-F.
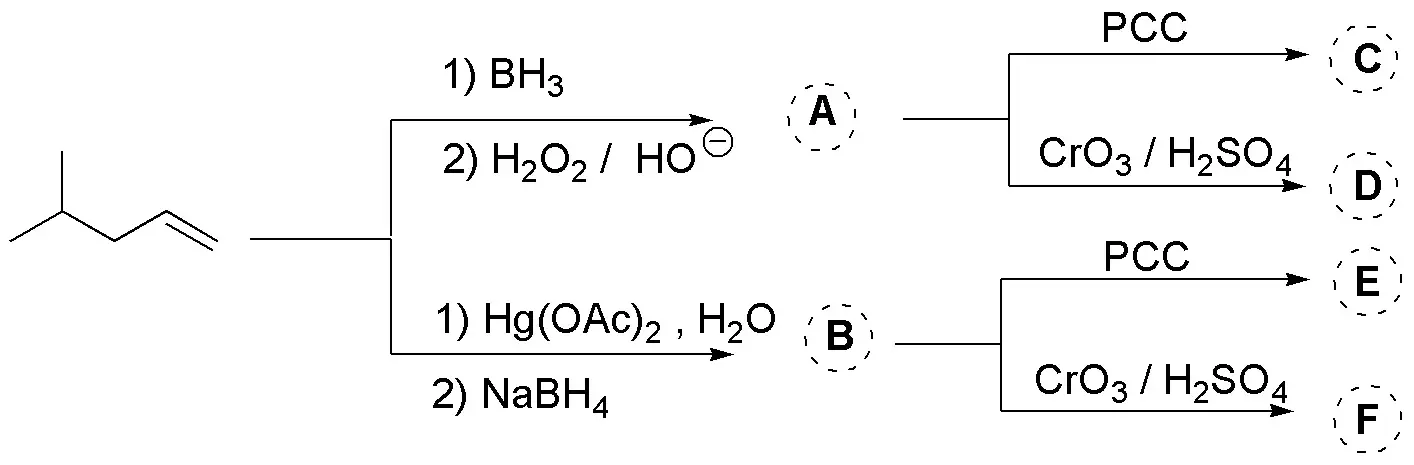
Problem 30)
Complete the scheme (A-G):
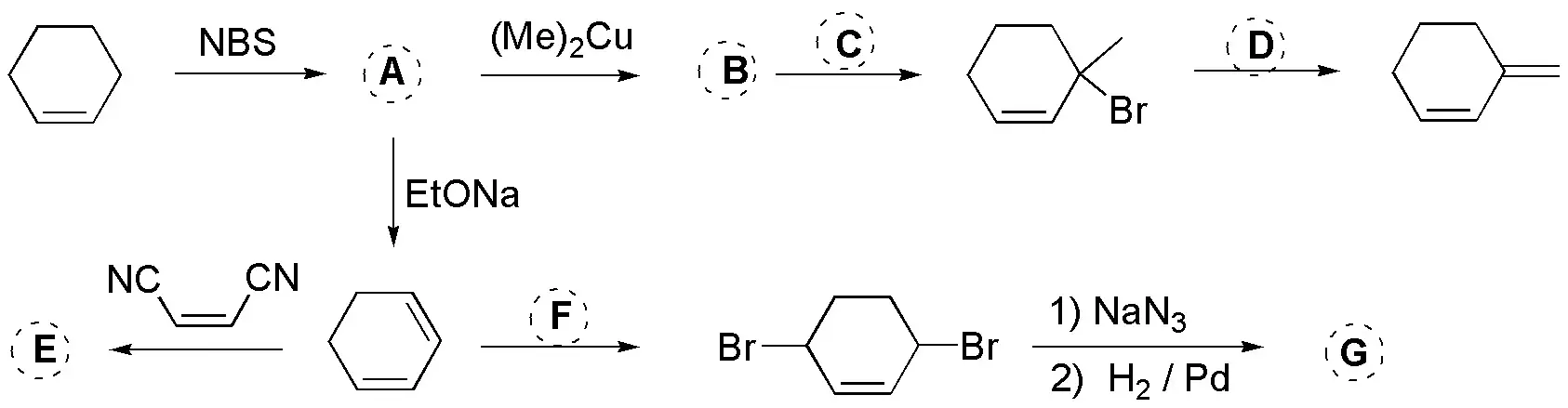
Problem 31)
Deduce the structures of the compounds (A-G) shown in the scheme, from the reactions that take place.
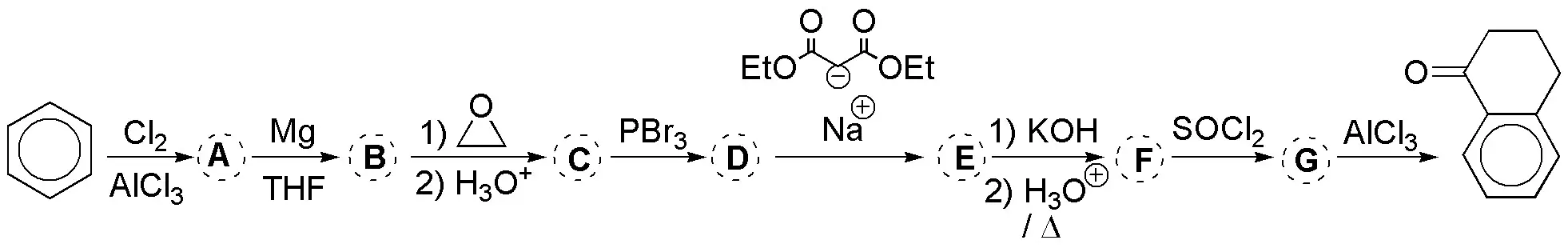
Problem 32)
Propose a procedure to perform the following transformations (a-d):
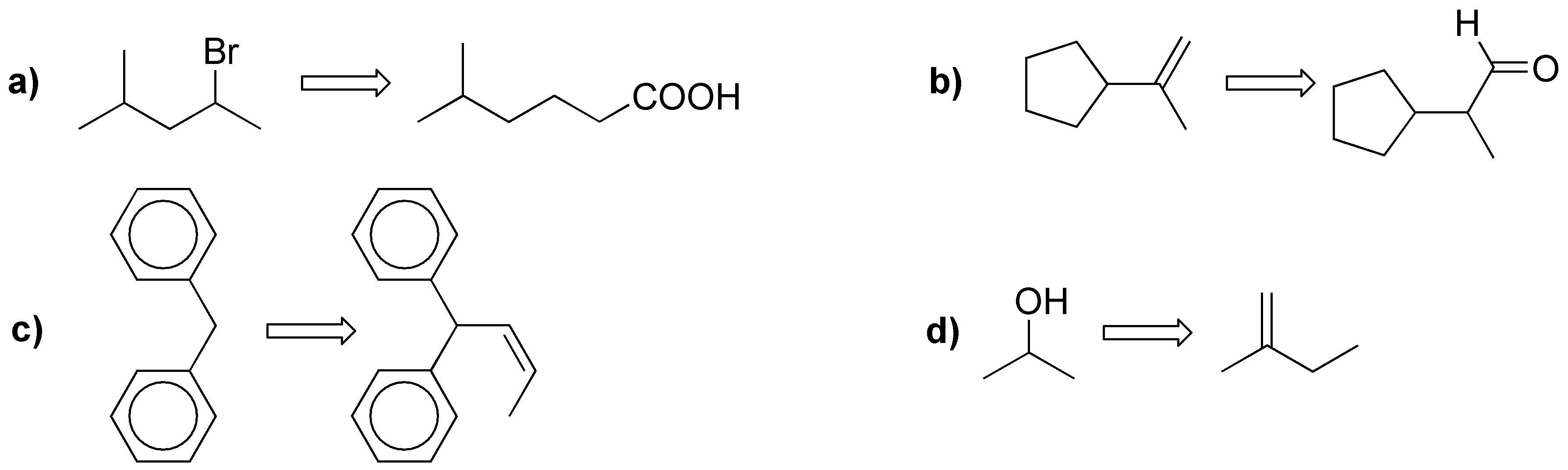
Problem 33)
Complete the scheme, describing the reactants A-G.
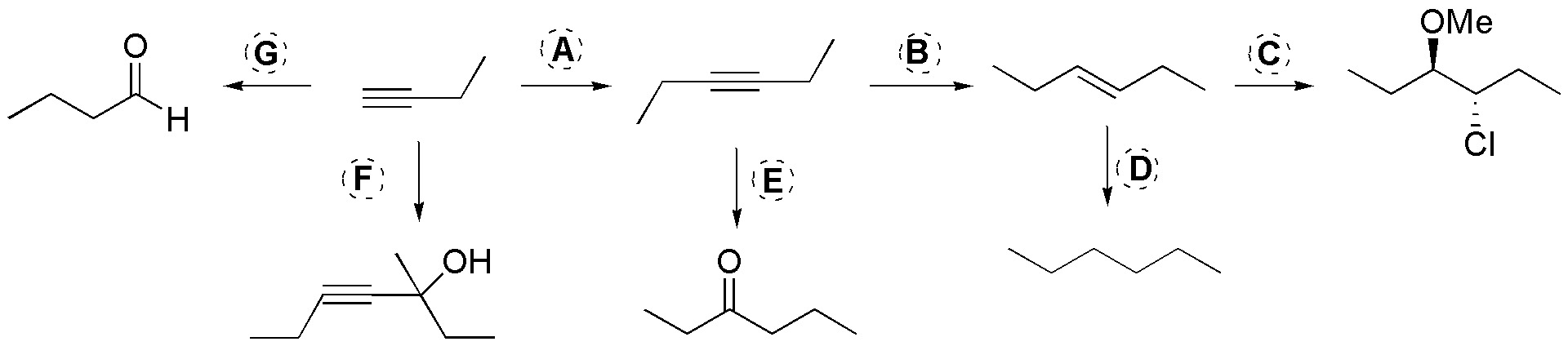
Problem 34)
Complete the following scheme (A-E), indicating the reagents needed for this purpose, and keeping in mind that transformations A and D require two steps.
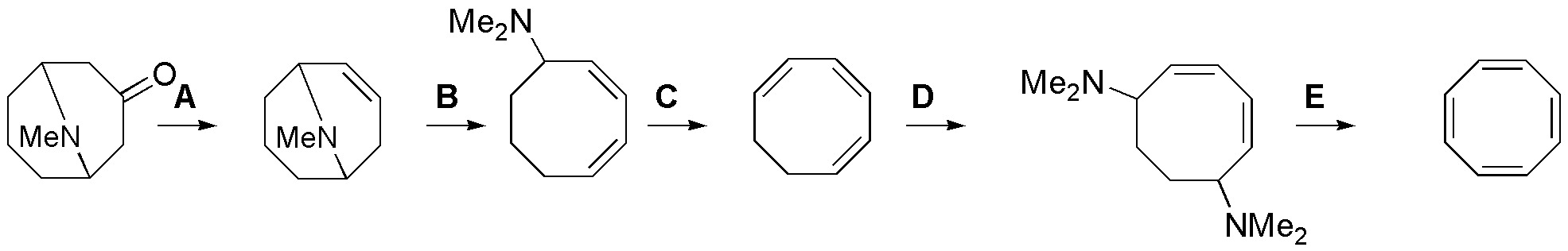
Problem 35)
Design a procedure to perform the following synthesis:
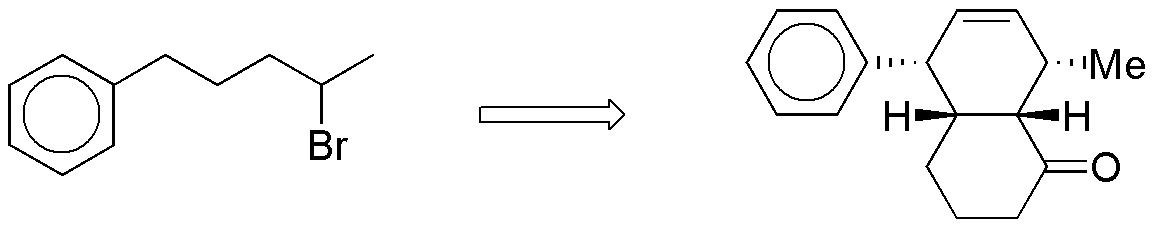
Problem 36)
Complete (A-D) the synthesis of 5-ethyl-6-methylhepta-1,6-diol from allyl bromide, according to the following scheme:
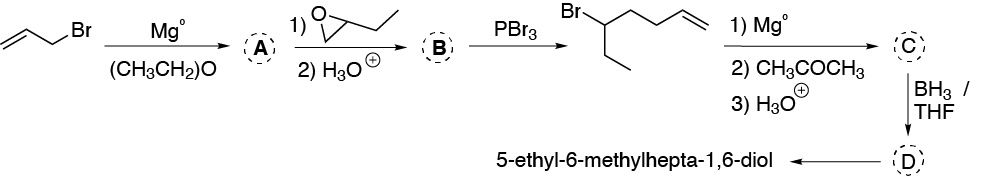
Problem 37)
Describe the reactions necessary to transform the reagent methyl 6-oxoheptanoate into the product 6-oxoheptyl benzoate.
Problem 38)
When acetic acid is treated with bromine in the presence of phosphorus, compound A is obtained. Reaction of A with NaCN / DMSO leads to the formation of B. If B is reacted with an aqueous mineral acid in the presence of ethanol C (C7H12O4) is obtained. If C is treated with sodium ethoxide in ethanol, followed by benzyl bromide leads to the formation of D (C14H18O4). Hot acid hydrolysis of D leads to E (C9H10O2). Deduce the structures of A, B, C, D and E.
Problem 39)
A compound A of molecular formula C7H14O can be obtained from B (C6H10O) or from C (C7H12) interchangeably. When it is obtained from B, lithium dimethylcuprate is needed, whereas if it is prepared from C it is necessary to use diborane, followed by H2O2 in basic medium. Determine the structures of A, B and C, knowing that B is synthesized from cyclohexene and peracetic acid.
Problem 40)
Bromobenzene reacts with magnesium in THF to give compound A. A is transformed by bubbling CO2 and treating with an acid, to give B (C7H6O2). Treatment of B with methanol in acid medium leads to C (C8H8O2). When C is reacted with 2 mol of A and subsequent acidification, D (C19H16O) is obtained. Deduce the structures A, B, C and D based on the reactions described.
Problem 41)
Design a synthetic route to perform the following transformation:
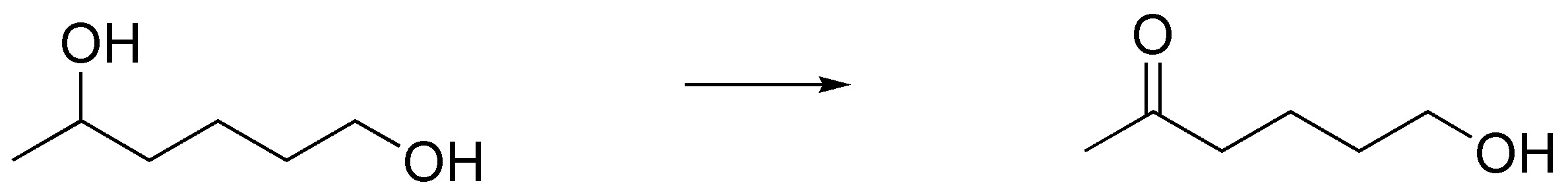
Problem 42)
Cyclopentene when treated with a mineral acid leads to the formation of a compound A which when the corresponding calculation is carried out, presents an unsaturation. When A is treated with potassium dichromate in an acidic medium B is obtained. The reaction of B with m-chloroperbenzoic acid leads to the formation of C. Deduce the structures of compounds A-C.
Problem 43)
Design a feasible procedure for the preparation of the following compounds (a-d) by malonic or acetylacetic and related syntheses.
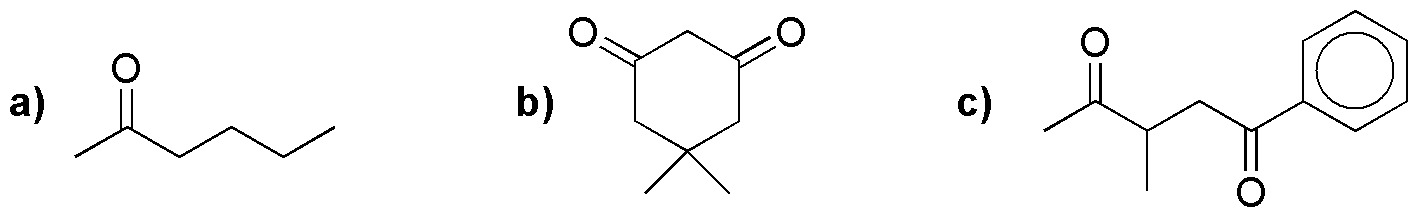
Problem 44)
The molecule known as disparlura is a pheromone of a moth species, the synthesis of which is partially described in the following scheme. Deduce the structure of the compound.
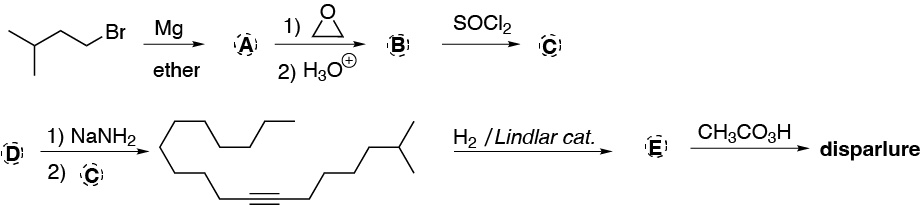
Problem 45)
From cyclohexanone, prepare the following compounds (a-d):
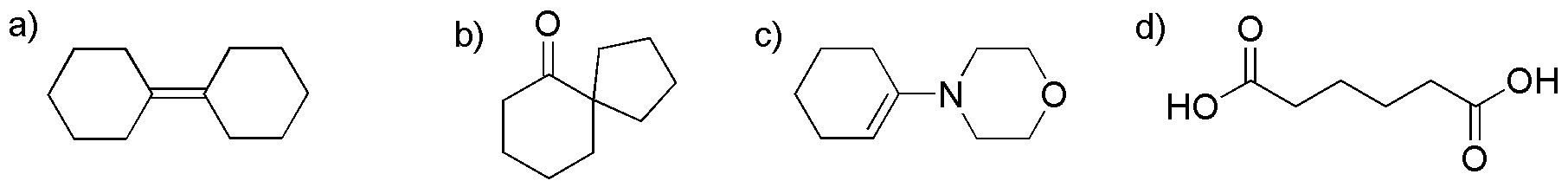
Problem 46)
Design a procedure for the preparation of the following compound from benzenonitrile.
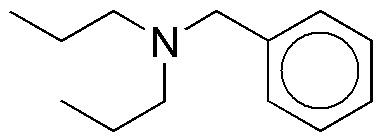
Problem 47)
Starting from cyclopentene, prepare compounds (a) and (b).

Problem 48)
Complete the scheme, describing the reagents A-F.