Written by J.A Dobado | Last Updated on May 2, 2024
What is liquid-liquid extraction?
Liquid-liquid extraction (abbreviated as extraction) is one of the most common basic operations in the organic chemistry laboratory and allows the isolation and purification of a product resulting from a chemical reaction.
Extraction can be defined as the transfer of a substance X from a liquid phase A to another liquid phase B. Both phases (or solvents) must be immiscible, so that they can thereby form two distinct phases and can separate.
The exchange of the substance X between the two phases A and B is given by the Nernst equation:
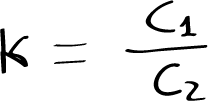
where C1 and C2 are the concentrations of substance X in B and A, respectively, and K is the partition coefficient, which depends on temperature.
This operation is typically performed between an aqueous solution (aqueous layer) and a water-immiscible organic solvent (organic layer) with the aid of an extraction funnel (separating funnel). The relative position of the two layers (upper, lower) depends on the density ratio. Chlorinated solvents such as chloroform (CH3Cl), methylene chloride (CH2Cl2), carbon tetrachloride (CCl4), etc., always remain in the lower layer, as they are denser than water.
However, other organic solvents have lower densities than water (diethyl ether, ethyl acetate, toluene, hexane, etc.) and therefore always remain in the upper layer.
Clearly, water miscible solvents are not useful for this process, e.g. acetone, MeOH, EtOH, etc.
Separatory funnel: liquid-liquid extraction is performed on a laboratory scale with a separatory funnel, a conical or pear-shaped vessel with a ground glass stopper at the top and a stopcock connecting the vessel to an outlet tube terminating in a narrowing.
Historical background
The history of liquid-liquid extraction, also known as solvent extraction or partitioning, can be traced back to ancient times when people used natural oils and fats to extract medicinal compounds from plants. The early forms of solvent extraction were based on the principle of like dissolving like, and were primarily used in the preparation of perfumes, dyes, and other aromatic compounds.
In the 20th century, liquid-liquid extraction underwent a period of rapid growth and innovation, fueled by advances in chemical engineering and process optimization. New types of solvents, such as ionic liquids and deep eutectic solvents, were discovered, and new techniques such as countercurrent extraction and membrane extraction were developed.
Today, liquid-liquid extraction is a mature and widely used technology with a diverse range of applications, from the separation of rare earth metals to the extraction of essential oils from plants. While the basic principles of liquid-liquid extraction have remained unchanged over time, advances in materials science, analytical chemistry, and computer modeling continue to drive new developments and innovations in this field.
How to use
For a successful extraction, the following steps are necessary:
- The separatory funnel is attached on a metal ring to the laboratory grid, placing a collecting vessel underneath. Generally an Erlenmeyer flask is used.
- The height of the separatory funnel is adjusted so that the outlet pipe is a few centimeters away from the collector.
- Check that the stopcock is closed before adding any liquid.
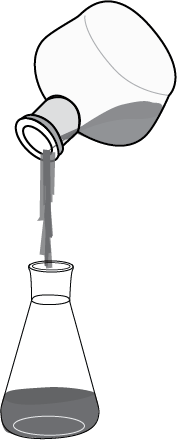
- Using a conical funnel, the two immiscible layers are poured into the extraction funnel (see Figure: steps one and two).
- Check that the stopper is properly tightened and closes properly without any leakage.
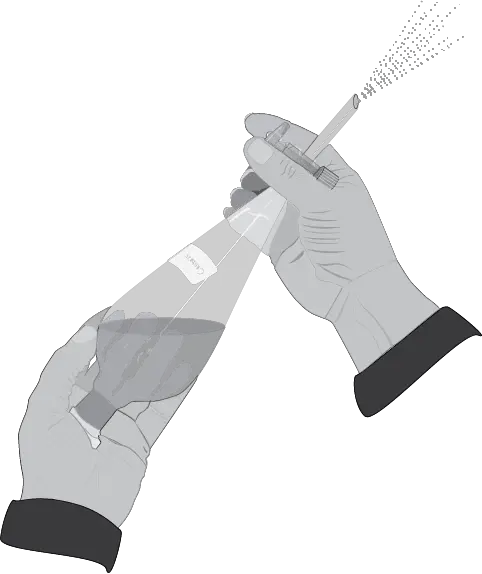
- Remove the separatory funnel from the metal ring and hold it firmly with the stopper side over the left (for right-handed) or right-handed (for left-handed) hand. With the free hand, hold the funnel between the fingers in the stopcock area, so that it can be opened and closed comfortably with the tip of the index finger and thumb (see Figure: step three).
- Then shake the funnel vigorously with both hands.
- It is important to open the stopcock occasionally to remove the excess pressure that sometimes builds up inside (do not direct the escaping gases towards you or anyone else).
- After shaking, put the funnel back into the metal ring and remove the stopper.
- Allow to stand until the two layers are separated (decanted).
- The layer remaining at the bottom is emptied by opening the stopcock to the boundary of the two layers.
- Finally, the top layer is poured into another vessel by grasping the funnel by the neck (see Figure: steps four and five).
General scheme
The mixture to be separated is added to a separatory funnel, and the aqueous layer is added first.
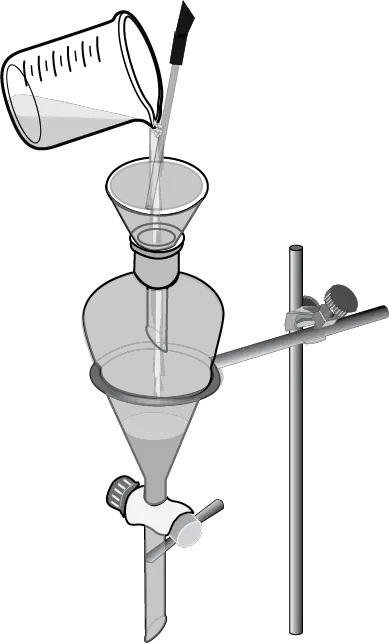
The organic solvent layer is then added to the funnel, forming two separate layers.
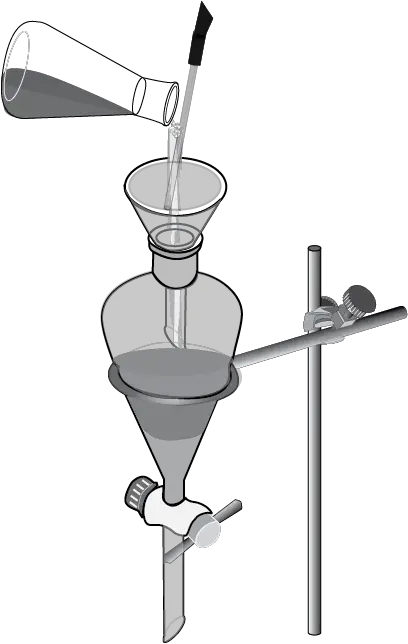
The mixture is stirred vigorously, and then the stopcock is opened slightly to release any overpressure that may have built up.
The separatory funnel is positioned over a collection vessel, and the stopcock is opened to allow the denser layer to flow out first.

After the bottom layer has been collected, the funnel is then tilted and the upper layer is poured out through the mouth of the funnel.
Overall, this technique allows for the separation of different compounds based on their solubility in the immiscible solvents used, providing a means for purification or isolation of specific compounds.
A desiccant may be added to remove any water that may have entered the organic layer.
Practical considerations
Sometimes, the separation between the two layers in the extraction process presents a number of difficulties that slow down the process, such as the formation of interfaces, foams or emulsions. There is no standard procedure to solve such problems. Thus, a simple quick rotation of the funnel is enough to break them.
In other cases, it is useful to add some salt crystals (NaCl) or a concentrated brine solution. But, in general, waiting is the most effective solution.
When the extraction is performed, the two layers are saturated with respect to the other solvent: water with organic solvent and the organic layer with water. Therefore, the water must be removed from the organic solvent to obtain the pure product by drying.
On the other hand, extraction is not always used simply to separate compounds by distributing them between organic and aqueous layers. Sometimes, it is useful to force extraction accompanied by a chemical reaction (most commonly through an acid-base reaction or through reactions that form metal complexes), allowing purification of the compound if it is contaminated with by-products of different chemical properties.
For example, in the case of aldehydes, such contaminants can be removed from an organic layer by washing with an aqueous bisulfite solution; alkenes can also be purified with silver salts or carboxylic acids with basic solutions, etc. This liquid purification process is called “solution washing” and is performed in the same way as a liquid-liquid extraction.
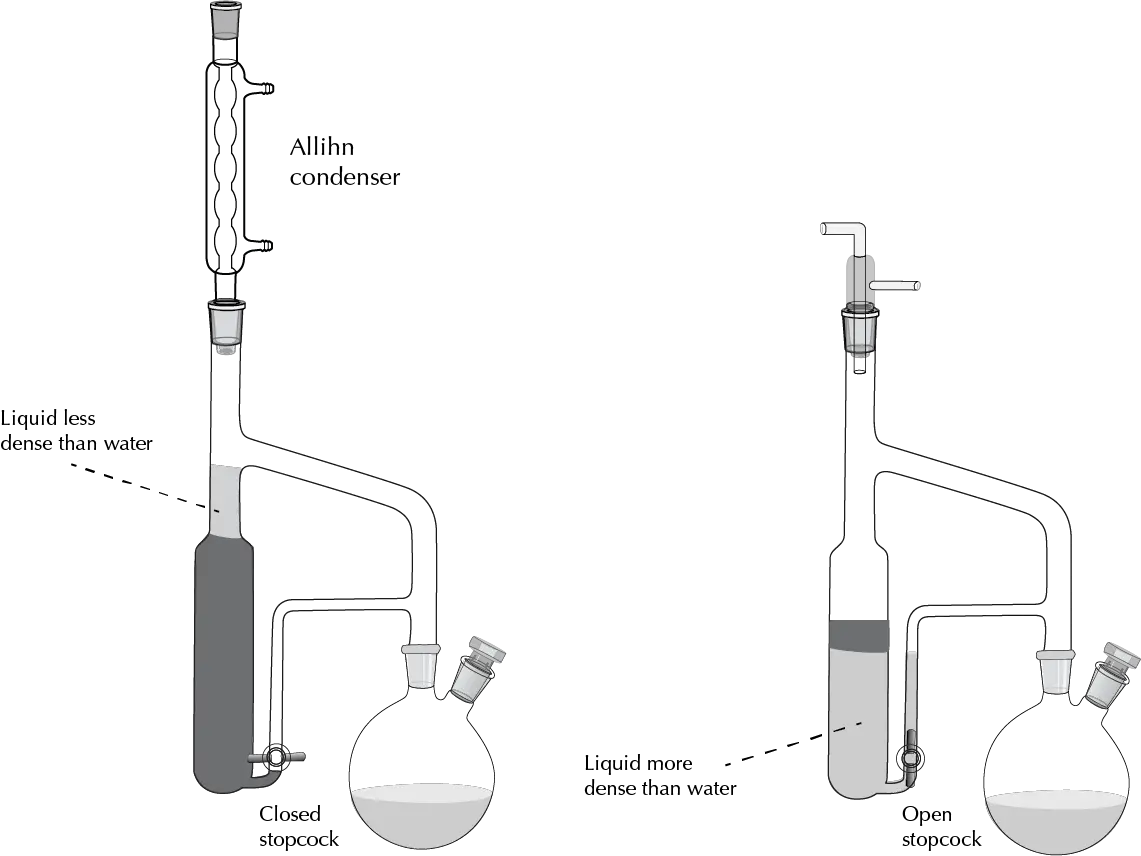
Continuous liquid-liquid extraction
When in a liquid-liquid extraction, it is not possible to isolate an organic product even using the strategies indicated above, because the partition coefficient is very unfavorable for the desired product, the solution would be to perform a large number of liquid-liquid extractions, which is not operative from the practical point of view. In such cases, we resort to continuous liquid-liquid extraction, which is illustrated in the following figure, taking into account the higher or lower density of the organic solvent with respect to that of water.
Advantages and disadvantages of liquid-liquid extraction
Overall, liquid-liquid extraction is a powerful and versatile method of separation and purification, but its effectiveness depends on careful selection of solvents and optimization of process conditions.
Advantages
- High selectivity: Liquid-liquid extraction is highly selective and can be used to isolate specific compounds from a mixture based on their physical and chemical properties.
- High efficiency: Liquid-liquid extraction can achieve high levels of separation and purification efficiency. It is also a relatively fast process and can be scaled up for industrial applications.
- Versatility: Liquid-liquid extraction can be used for a wide range of applications, from separating and purifying natural products to recovering precious metals from electronic waste.
- Cost-effective: Liquid-liquid extraction can be a cost-effective method of separation, especially when compared to other techniques such as chromatography.
Disadvantages
- Limited solubility: The solubility of the compound being extracted in the solvent is critical to the success of liquid-liquid extraction. If the compound has low solubility in the solvent, the process may not be effective.
- High solvent consumption: Liquid-liquid extraction can require large amounts of solvents, which can be expensive and environmentally unfriendly.
- Emulsion formation: Emulsions can form during the extraction process, which can make it difficult to separate the two phases and recover the desired compound.
- Safety concerns: Some solvents used in liquid-liquid extraction can be hazardous to health and the environment, requiring special handling and disposal procedures.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2