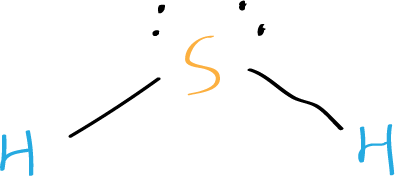Written by J.A Dobado | Last Updated on May 2, 2024
What is the Lewis structure of hydrogen sulfide H2S?
Hydrogen sulfide H2S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. To draw the Lewis structure of hydrogen sulfide, follow these step-by-step instructions.
How to Draw the Dot Structure for H2S
Step 1: Count the total number of valence electrons
Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide, add the number of valence electrons in each atom. Hydrogen has one valence electron, while sulfur has six valence electrons. Therefore, H2S has a total of eight valence electrons.
2×(1) + 6 = 8
Step 2: Determine the central atom
In hydrogen sulfide, sulfur is the central atom, since it is the atom with the highest valency. Hydrogen can only form one bond, while sulfur can form up to six bonds, so sulfur is capable of accommodating two hydrogen atoms.
Step 3: Connect the atoms
Draw the central sulfur atom and the two hydrogen atoms around it. Connect each hydrogen atom to the sulfur atom with a single bond. This will use up two valence electrons, leaving six more.
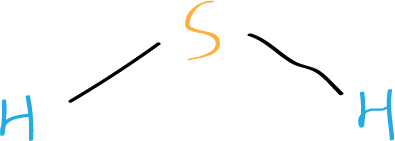
Step 4: Place the remaining electrons
Place the remaining six valence electrons around the sulfur atom in pairs. Sulfur will have two lone pairs and two bonding pairs of electrons. Lone pairs are non-bonding pairs of electrons.
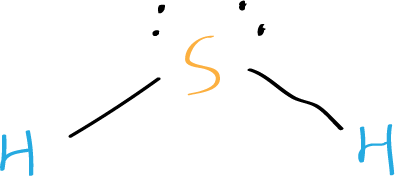
Step 5: Check the octet rule (formal charges)
In the Lewis structure of hydrogen sulfide, sulfur has eight electrons around it, including two lone pairs and two bonding pairs. Each hydrogen atom has two electrons around it.
The formal charge is a measure of the distribution of electrons in a molecule. It is calculated by subtracting the number of non-bonding electrons and half of the bonding electrons from the number of valence electrons in an atom. The formal charge of an atom should be as close to zero as possible. To check the formal charges in water, we use the following formula:
Formal charge = Valence electrons – Non-bonding electrons – 1/2 (Bonding electrons)
For the sulfur atom, the formal charge is:
6 – 4 – 1/2(4) = 0
For each hydrogen atom, the formal charge is:
1 – 0 – 1/2(2) = 0
This satisfies the octet rule, which states that atoms tend to bond in such a way that each atom has eight valence electrons around it.
Step 6: Draw the final structure
The final Lewis structure of hydrogen sulfide looks like this: