Written by J.A Dobado | Last Updated on April 22, 2024
What are inert atmosphere reactions?
There are reagents or products that are sensitive to humidity, oxygen in the air and even CO2. In these cases, effective protection of reactions in an inert atmosphere is necessary. This is because the yields can be affected by the partial or total destruction of a sensitive substance during the reaction. Also, some of these undesired transformations can become violent, leading to explosions or fires. For all these reasons, reactions of this type are among the most complex processes that can occur in an organic chemistry laboratory. Thus, it is important to deal with them once the main basic laboratory operations are known.
With relatively simple guidelines, it is possible to maintain an inert atmosphere inside an assembly using conventional labware.
There are several aspects to consider:
- Glassware must be perfectly dry.
- Solvents must be anhydrous.
- Inside the assembly, the air must be replaced by an inert gas, such as nitrogen or argon. Therefore, the reaction must be carried out under a constant flow of inert gas, or at least the air must be displaced with an inert gas and the interior must be maintained with a slight pressure of the inert gas.
Precautions with the material
Glassware should be perfectly dry and should be used immediately after removal from the stove. It may even be convenient to treat the various components of the assemblies with a hot air dryer while they are being assembled, to prevent moisture accumulation.
- Solvents: A first consideration to take into account, is that in this type of reactions the solvents must be anhydrous. That is to say, totally free of moisture. For this, the solvents must be of high quality and receive a special treatment for the complete elimination of traces of water. Although there are many suppliers who market solvents packaged as “anhydrous”, the removal of seals and exposure to the atmosphere, however minimal, may invalidate their use for some particularly sensitive reactions. In these cases, it is required that the “anhydrous solvent” condition be achieved immediately prior to use.
Using anhydrous solvents
There are basically two procedures to obtain anhydrous solvents:
Individualized chemical treatments
Drying of the most common solvents:
- THF (tetrahydrofuran): It is first treated with calcium hydride or 4 Å molecular sieves and then heated under reflux under inert atmosphere in the presence of spun sodium and benzophenone for several hours until the solvent turns deep blue. This indicates that the solvent is dry. From this point on, the required amount can be taken. If the mixture turns orange, this indicates that a new quantity must be prepared.
- Diethyl ether. It dries in the same way as THF. For this type of solvent, a setup is usually used in which refluxing is carried out with the drying agent until the colorimetric indicator shows that the solvent is free of water. At this point, the solvent can begin to be collected in a flask that acts as a reservoir by operating a tap system. If not used, the solvent can be returned to the flask where refluxing takes place so that it remains in contact with the desiccant. The whole process is carried out under inert atmosphere.
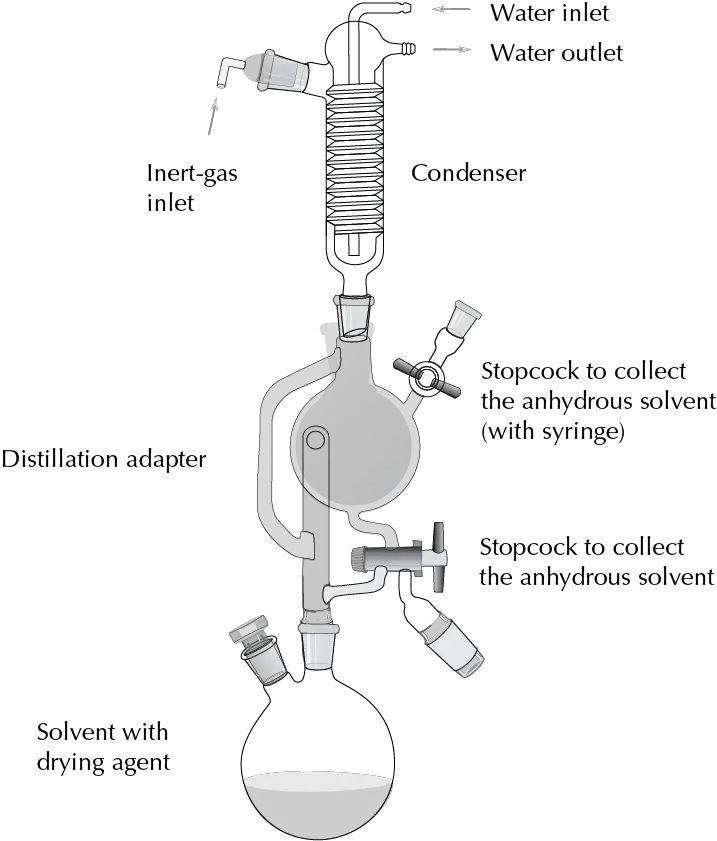
| DANGER! “To remove the sodium waste from this procedure, EtOH is slowly added. This process is slow and is facilitated by occasional shaking of the flask. Once it is certain that no more hydrogen is given off, small amounts of 1:1 hydroalcoholic mixtures can be added very slowly. When it is observed that there is no further reaction, water is added until the formation of two distinct phases which should be separated and poured into the appropriate waste container.” |
- CH2Cl2: It is dried with calcium hydride, and then distilled over this same compound. There is no colorimetric indicator that the solvent is dry.
- MeOH: For most uses, it is dried on 3 Å molecular sieves overnight and then distilled. Alternatively, MeOH can be dried with magnesium methoxide. For a volume of 1 l, 100 ml of MeOH dried by the above procedure is taken and refluxed with magnesium chips (5 g) and iodine (0.5 g) until all the magnesium has been reacted. MeOH is added to a volume of 1 l and kept at reflux for 2-3 h under inert atmosphere.
- MeCN: Stirred with 4 Å molecular sieves, and distilled over calcium hydride.
| DANGER! “The process of elimination of magnesium or calcium hydride is similar to that used for sodium. When hydrogen release ceases upon addition of EtOH, water can be added with caution until no bubble formation is observed. If an aqueous phase and an organic layer are formed, they should be separated and poured into the appropriate waste container. If the water and solvent are miscible, add more water and pour into the appropriate waste container.” |
- DMF (N,N-dimethylformamide): It has the disadvantage of decomposing slowly at room temperature and more rapidly at reflux, giving dimethylamine and CO. The decomposition is catalyzed by trace acids and bases or with basic drying agents such as calcium hydride. It can be dried with barium oxide or 4 Å molecular sieves at room temperature overnight, the drying agent being removed by decantation and distillation under vacuum ∼ 20 mmHg is a sufficient vacuum to reduce the boiling point further of DMF).
For long-term storage of anhydrous solvents it is convenient to store them in a container on 4 Å molecular sieves.
Any reaction or distillation must be carried out under inert atmosphere.
Dispensers for solvents
Anhydrous solvent dispensers are becoming more and more common in organic chemistry laboratories. High quality solvents are used, which are stored in metal safety containers and passed through columns with a specific filling that retains water or other residual molecules such as alcohols or amines, before exiting through the corresponding valve. This process is carried out by dragging the solvent through a stream of inert gas. The valves allow the filling of flasks of all types without contact with air.
Origin of inert gas
Gass cylinder or centralized circuit
Inert gases may be stored in a gas bale or available in a centralized system. In either case, the gas pressure is regulated by a pressure regulator, which is a device that reduces the pressure of a fluid and maintains the output flow at a fixed or selected constant pressure.
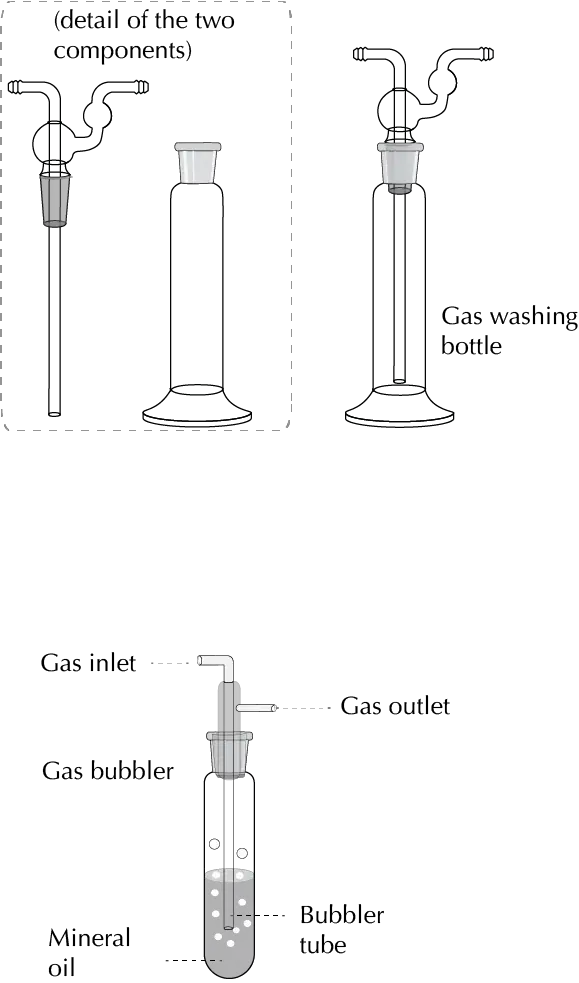
To control the flow rate of inert gas in an assembly, a bubbler is used at the end of the assembly. A bubbler is a glass device containing glycerin or a mineral oil through which the gas passes. The flow rate is regulated according to the bubbling of the gas in the fluid.
Using a balloon
A rubber balloon filled with inert gas connected to a syringe with needle is punctured in a septum, thus maintaining a slight pressure inside with the inert gas.
Transfer of reagents and solvents sensitive to air or humidity
In reactions under inert atmosphere it is common to handle reagents that are sensitive to moisture or oxygen in the air. There are several ways to transfer these reagents to the reaction vessels. Some of these ways are described below.
First of all, for liquid reagents in solution, the bottles are sealed by a double closure consisting of a metal capsule similar to that of a soft drink bottle with a hole in the center that exposes a small surface of a polymeric material (such as polypropylene or similar) that can be pierced by needles or cannulas repeatedly without the reagent coming into contact with the air. For conventional bottles a septum can be used as a stopper.[1]
A simple syringe with a needle can be used to take the quantities required. To facilitate the filling of the syringe and to avoid the entry of moisture or air, it is convenient to puncture another needle attached to a flexible tube with nitrogen or argon. The increased pressure of the inert gas drives the liquid to fill the needle.
In order to transfer larger quantities, a cannula can be used, which is a steel tube with a needle termination at both ends. The container with the reagent or solvent is punctured with a flexible tube with inert gas and the cannula at one end and at the other end it is punctured into a flask with a septum and a gas outlet, such as another needle or even a bubbler. As the inert gas pressure in the reagent or solvent vessel increases, it passes through the cannula into the flask. If the inert gas no longer passes through, the transfer ceases.
Video on inert atmosphere reactions
References and notes
- [1] The septum seal can be secured with Parafilm® as it is ideal for sealing tubes, flasks, petri dishes and beakers among other containers. It is durable, resists moisture and adheres to different surfaces.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2