Introduction
The International Union of Pure and Applied Chemistry (IUPAC) is the body that issues nomenclature recommendations for organic and inorganic compounds, so that the scientific community can communicate without difficulty when using known or new compound names.
The recommendations of the nomenclature have been developed over the years through various committees that continue to make recommendations and adjustments to meet the needs of naming of new substances and materials as they are discovered, constituting what is called systematic nomenclature.
Nomenclature recommendations, for organic compounds, can be grouped into two major sets published[1] in 1979 and 1993.
For students of Organic Chemistry, the direct consultation of these recommendations can be confusing, given their complexity, so it is necessary to order them, interpret them and present them with a minimum systematics that allows their correct learning. On the other hand, it is essential to carry out the necessary translation into the corresponding language, making the rigor of the standard compatible with the principles of Grammar.
In this section, we address the basic recommendations that allow you to formulate and naming organic compounds the most common.
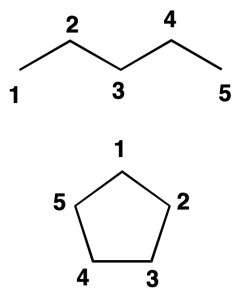
Locator
As a concept prior to the development of the nomenclature of organic compounds, it is convenient to define the term locator. In chains and cycles, Arabic numbering is used in a correlative manner to indicate the position of a particular atom belonging to that chain or cycle. This number is called locator.
Systems of nomenclature
There is no single way to name molecules. On the contrary, the IUPAC allows several systems for naming an organic compound (recommendation C-0). The vast majority of substances, which appear in general organic chemistry texts, can be named following two nomenclature systems:
Naming a substitute
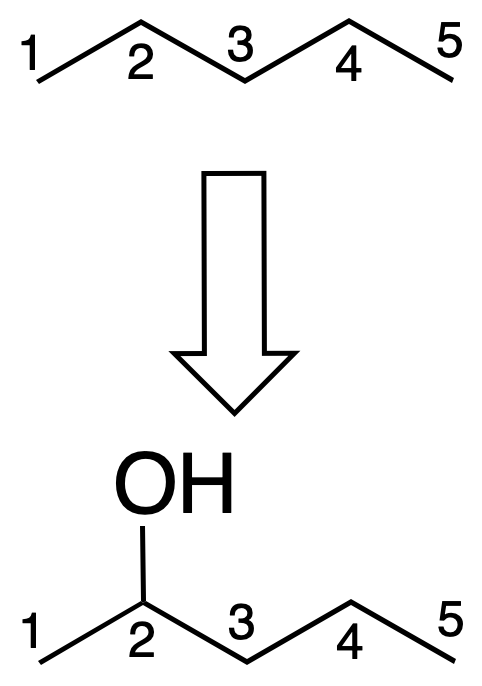
It is considered that a hydrogen in the structure hydrocarbon (only carbon and hydrogen) is replaced by the corresponding function. The name is constructed with the hydrocarbon of the same number of carbons, followed by the name of the function. E.g.:
- As the main function: the hydrogen in the 2-position of pentane is replaced by an –OH group. The name is constructed from the starting hydrocarbon (pentane) plus the suffix describing the function (-ol), i.e. pentan-2-ol.
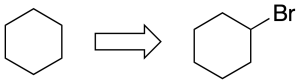
- As a substituent: the bromo function replaces a hydrogen in cyclohexane and the name is composed of the prefix describing the substituent bromo, followed by the hydrocarbon of the same number of carbons, i.e. bromocyclohexane.
Nomenclature radical-function
It uses the name of the function next to the radical to which it is attached.
For example: in the molecule shown in the figure, the amine function is attached to a propyl radical[2] (CH3-CH2-CH2-). The name is constructed from the propyl radical, followed by the amine function (propylamine).
![]()
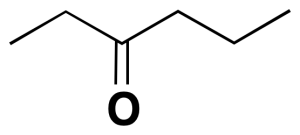
In the following molecule, the ethyl (CH3-CH2-) and propyl (CH3-CH2-CH2-) radicals are attached to the ketone function (C=O), so the name is composed from the alphabetically ordered radicals, ending with the name of the function (ethylpropylketone).
Sometimes, the choice of one or another system of nomenclature is the same, on the contrary, in other occasions, it is applicable to a single form. Generally, it is preferred to the naming a substitutebut you must choose the simpler procedure.
Naming compounds mono-functional
Learning the formulation and nomenclature of organic compounds begins with the simplest cases in which the molecule has only one functional group. The following Table summarizes the IUPAC rules for a selection of the most significant functional groups in organic chemistry.
- Alcohols
- Aldehydes
- Alkanes
- Alkenes
- Alkynes
- Amides
- Amines
- Anhydrides
- Aromatic compounds
- Azides
- Carboxylic acids and their salts
- Cyanates and isocyanates
- Esters
- Ethers
- Haloalkanes
- Halides-acid
- Halides-aryl
- Hydrazines
- Hydroxylamine
- Ketones
- Nitriles
- Nitroso and nitro compounds
- Peroxyacids
- Sulfenic, sulfinic and sulfonic acids
- Sulfides
- Sulfoxides and sulfones
- Thiols
Naming of compounds, polyfunctional
In the previous sections, it covers the recommendations for the formulation of compounds mono-functional. However, it is very common that an organic molecule present more of a functional group.
To address this issue, the IUPAC establishes a series of recommendations that allow these polyfunctional compounds to be named systematically.
Main function
In the first place, the IUPAC establishes a priority order for the functions, so that the highest priority is called main functionconsidering the rest as substituents. In the Table-A2 in the Appendix, establishes the order of priority of the most important functions, as well as its name when it acts as a substituent (prefix-) or as a function main (-suffix).
The groups described below (always behave as substituents, thereby using only the corresponding prefixes:
Function | Prefix | Function | Prefix |
-F | fluoro- | -IO2 | yodil- |
-Cl | chlorine- | -N3 | azido- |
-Br | bromo- | -NO | nitroso- |
-I | iodine- | -NO2 | nitro- |
-ClO | clorosil- | =N2 | diazo- |
-ClO2 | cloril- | -OR | (R)-oxy |
-ClO3 | percloril- | -SR | (R)-sulfanyl |
-IO | yodosil- |
A systematic name to an organic compound
From the structure of a compound, it is possible to assign a name by following a systematic summed up in 2 steps:
Step-1. Identification of the main function in accordance with the Table A2 of the Appendix.
Step-2. Identification of the main structure.
In systems acyclic the main structure is chosen according to the following criteria:
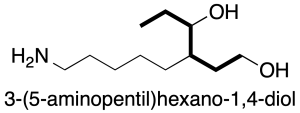
a) the chain with the highest number of main function units. Note that in the compound in the figure, although the longest chain has 9 carbons, the one highlighted in bold with 6 carbons is chosen because it has 2 OH groups of higher priority.
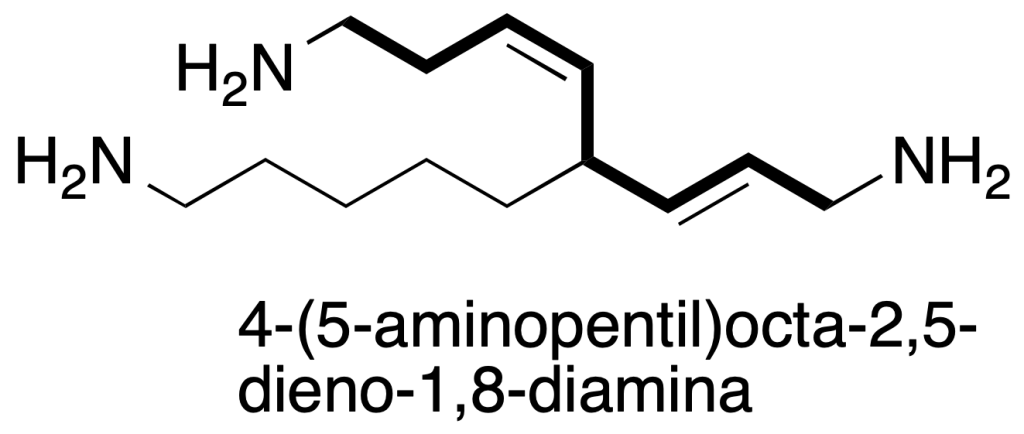
b) the chain that meets the above requirement and also contains as many double and triple bonds as possible. As shown in the figure, although there is a chain with 10 carbon atoms, two amino groups and a double bond, the one with 8 carbons highlighted is chosen because it has two double bonds.
c) the string of maximum length. If the above criteria are equal, the longest string is chosen.
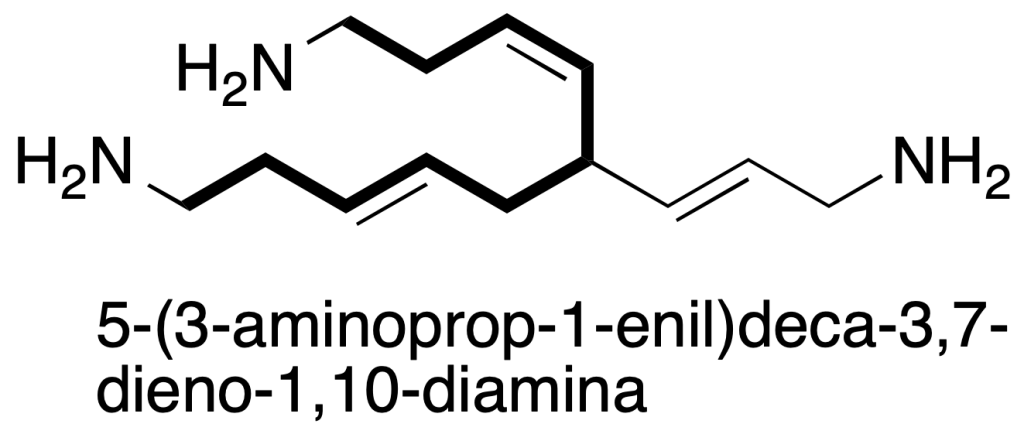
If you arrived to paragraph (c) none of the above criteria serves us using the following:
- Lower rates for the major groups.
- Lowest rates for multiple links.
- Maximum number of substituents cited as prefixes.
- Lowest rates to all of the substituents on the main chain cited as prefixes.
- The substituent cited first in alphabetical order.
- Lowest rates to the substituent cited first in alphabetical order.
References and notes
[1] published in 1979, is found in the book “Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H” Pergamon Press, 1979. Published in 1993 can be found in “IUPAC, Commission on Nomenclature of Organic Chemistry. A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993)” Blackwell Scientific publications, 1993.
[2] Radical, in this case, it is understood as a synonym for group or substituent of a molecule.