Written by J.A Dobado | Last Updated on April 22, 2024
What are organometallic compounds?
Organometallic compounds are substances in which an organic functional group is directly bonded to an R-M metal. Organometallic chemistry begins in the early 20th century.
Because metals are electropositive, carbon metal bonds (C-M) exhibit a high degree of ionic character. Therefore, dipolar resonance structures contribute significantly to the structure of these compounds.
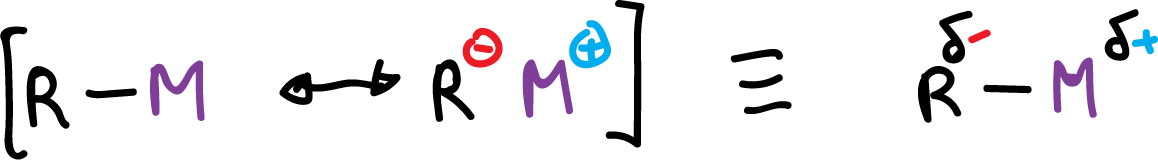
This bond polarization in the organometallic compound makes them synthetically useful as nucleophiles in addition compounds.
Other organometallic compounds, the organolithiums, started to be used after 1960-1970, due to a technical difficulty since their preparation requires inert atmospheres and totally dry environments.
In the middle of the 20th century, a great development began in the chemistry of other organometallics, such as palladium (Pd) and copper (Cu).
Palladium catalyzes any type of organic reaction.
Nomenclature
They are named by prefixing the metal name with the name of the corresponding functional group.
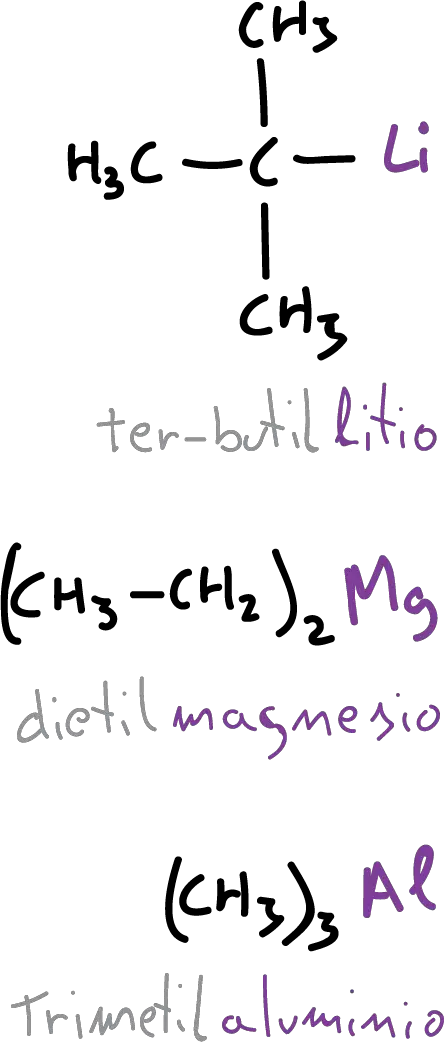
The compounds of boron, tin and silicon are also named as derivatives of the simple hydrides. In the case of tin, it can be named either stannane or tin.

In some organometallic compounds, not all valences of the metal are used to bond to the carbon, but include bonds to other inorganics. These compounds are named as organic derivatives of the corresponding inorganic salt.

Compounds of type R—MgX are known as Grignard reagents. They are widely used in organic synthesis.
Physical properties
Most organometallic compounds decompose in water, but are soluble in various inert aprotic organic solvents. Typical solvents used with these compounds are ethers and alkanes.
Preparation methods
Reaction of an alkyl halide with a metal
This method is most commonly used for the preparation of organolithium and organomagnesium compounds.
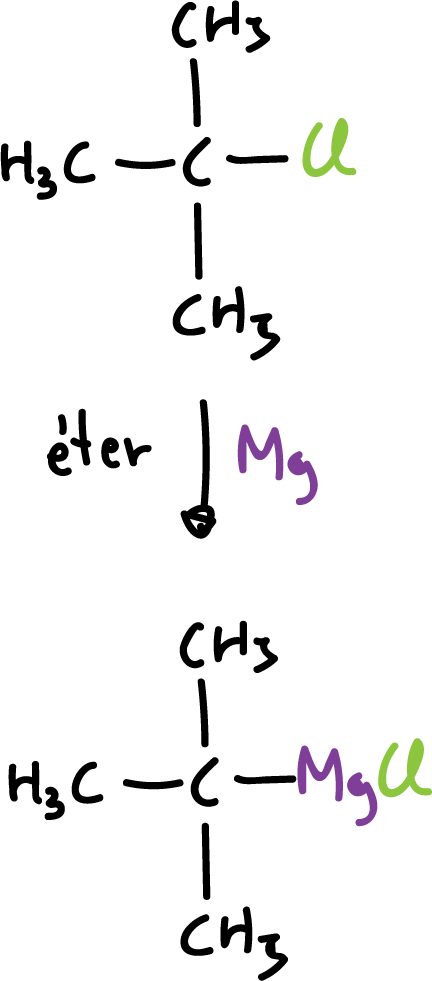
This reaction is an example of a heterogeneous mechanism, i.e., one that occurs at the interface between two distinct phases. Alkyl halide in solution must react with magnesium on the surface of solid magnesium. In this type of reaction, the surface area and its character are of great importance.
The reaction mechanism can be simplified as follows.
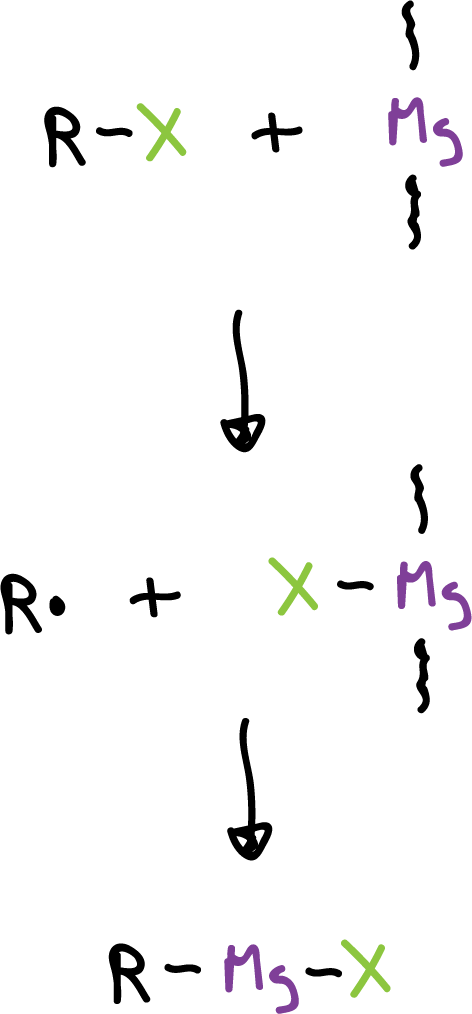
Although side reactions take place between organic radicals, however, the yields of R-MgX are high.
Alkyl lithium compounds are prepared in the same way.
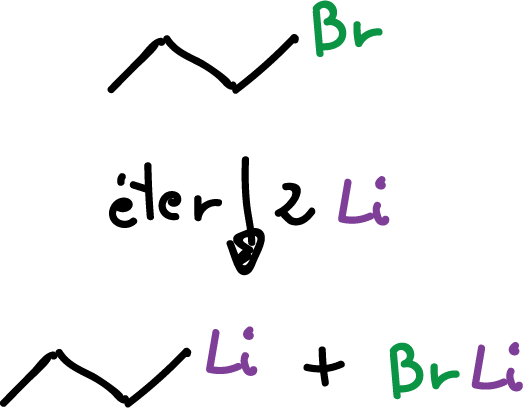
The compounds organosodium and organopotassium are not prepared in this way, since the main product results in alkane.
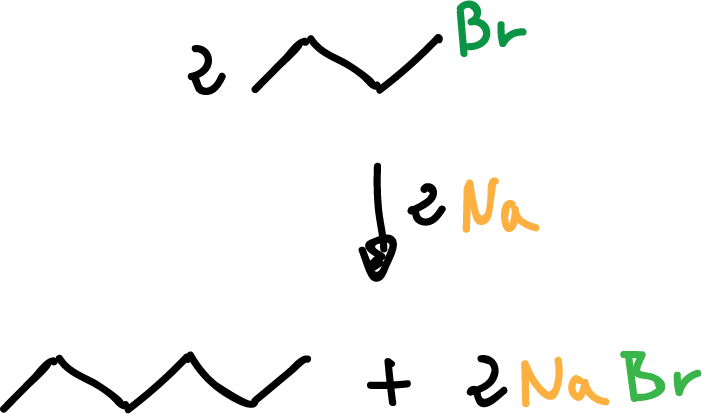
The alkane may result when the initially formed alkyl sodium or alkyl potassium displaces the halide of an unreacted alkyl halide molecule.
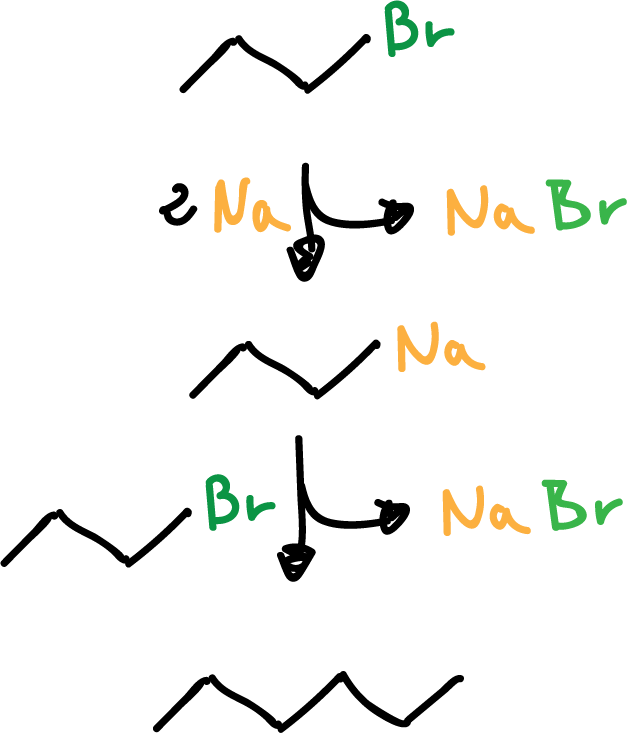
Reaction of organometallic compounds with salts
This is a reaction of an organometal with a salt to obtain a new organometal and a new salt.
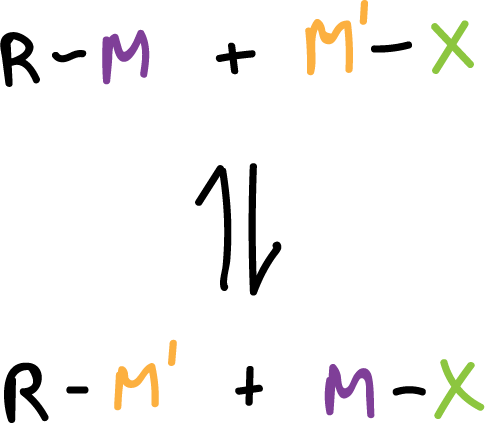
For example:
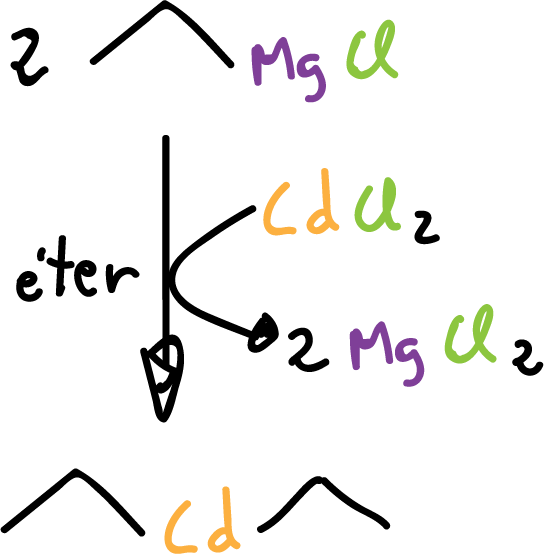
or to prepare tetraalkyl-silane compounds
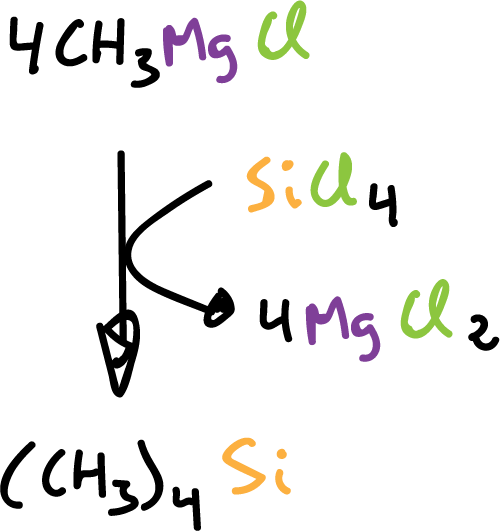
or dialkyl mercury compounds.
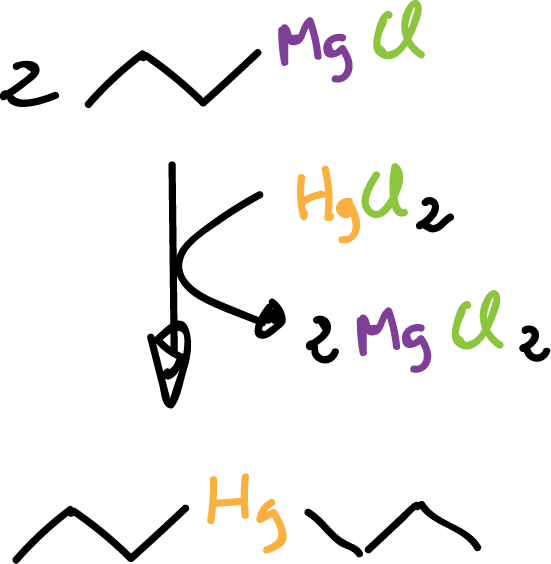
Other preparation methods
Organometallic compounds can be prepared by addition reactions to alkenes.
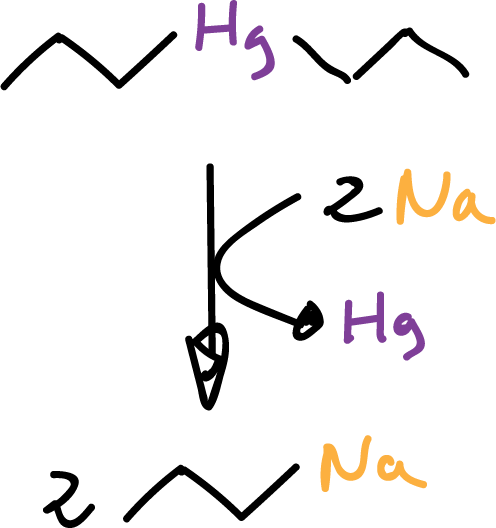
Other organometallics can be obtained by treating other organometallics with free metals,
or by disproportionation of two organometallic compounds.
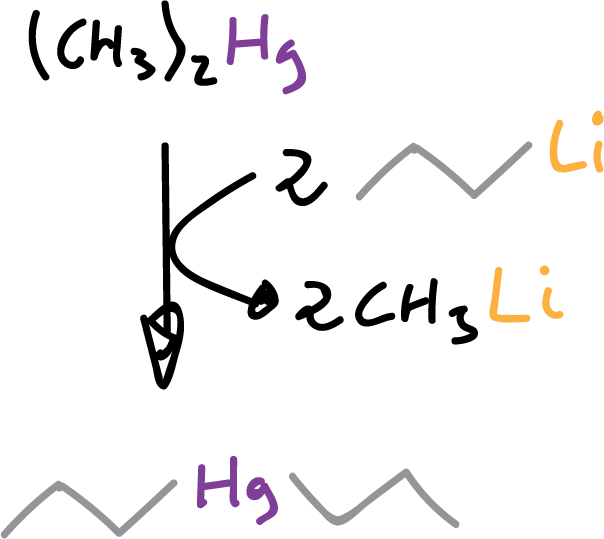
Reactivity of organometallic compounds
Hydrolysis
Organometallic compounds in which the metal has an electronegativity value of about 1.7 or less react with water to give the metal hydrocarbon and hydroxide.
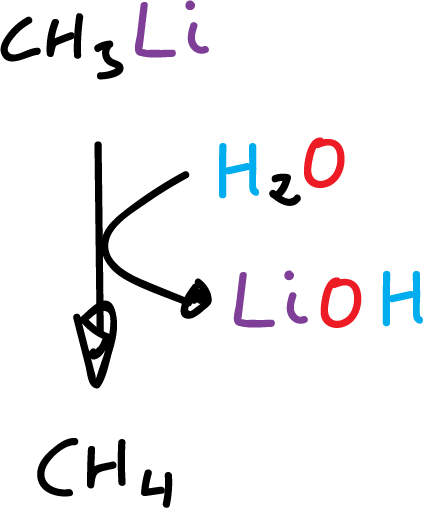
The more electropositive the metal (better electronegativity value) the faster the hydrolysis takes place.
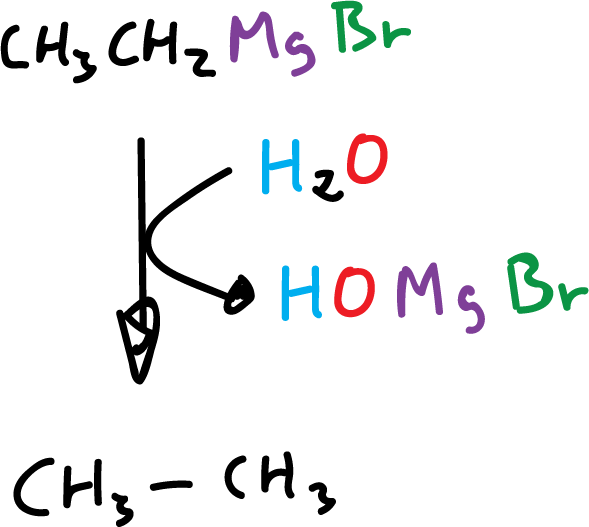
Alkyl-lithium, alkyl-magnesium, and alkyl-aluminum react violently with water.
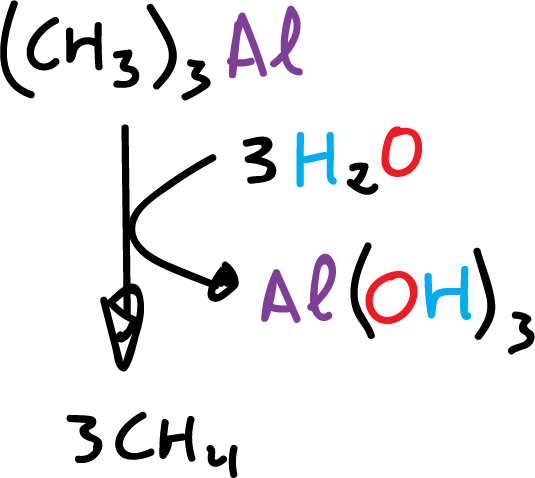
These compounds react similarly to other hydroxyl compounds, such as alcohols and carboxylic acids.
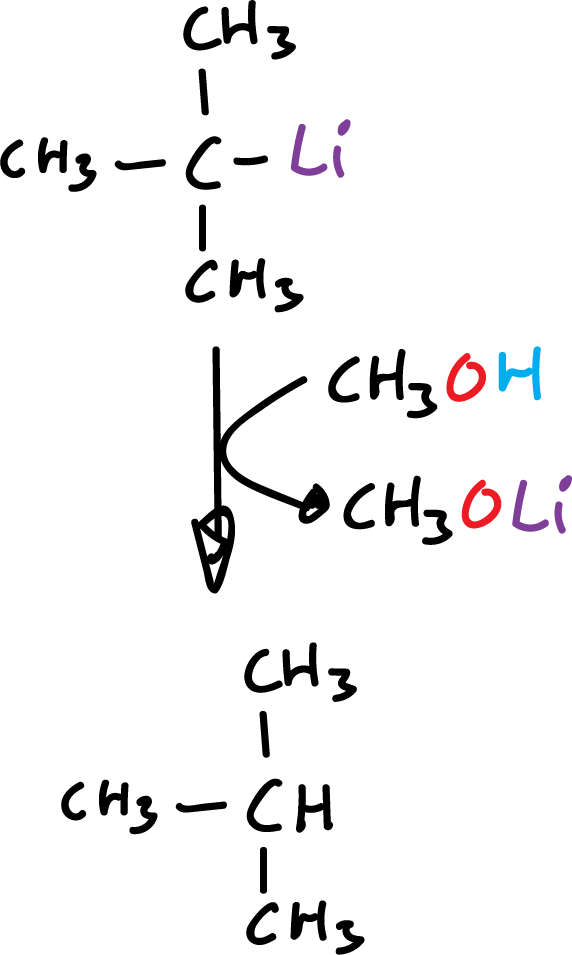
An important application of hydrolysis reactions is specific deuteration. When hydrolysis is carried out with deuterated water (deuterium oxide, D2O), the product is an alkane containing a deuterium in the position previously occupied by the metal.
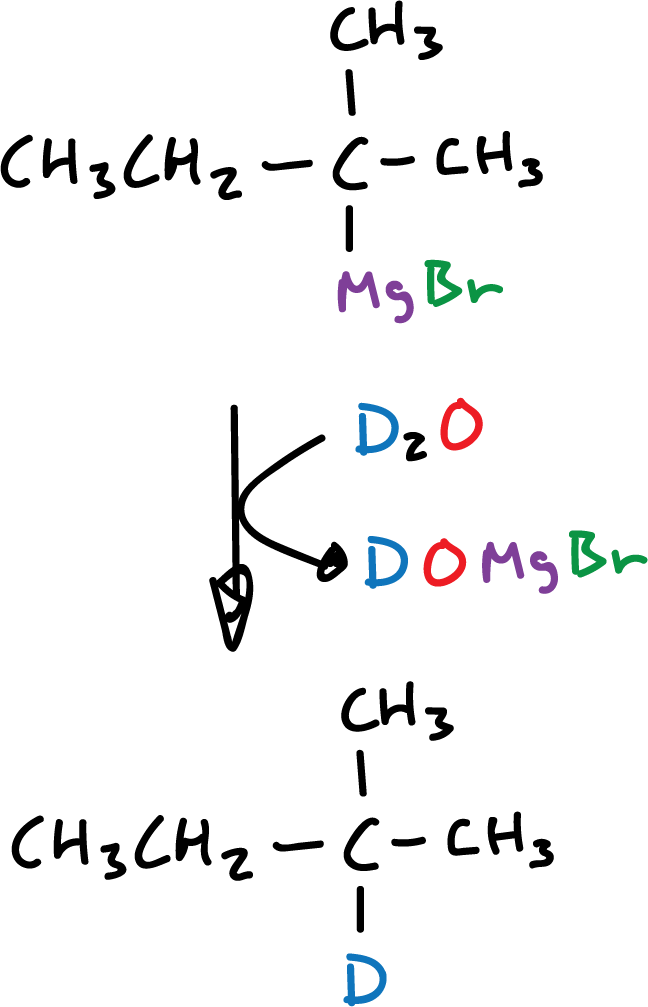
This reaction is a very versatile means to elaborate deuterium-labeled hydrocarbons in a specific position.
Reaction with halogens
Most organometallic compounds react vigorously with chlorine (Cl2) and bromine (Br2).
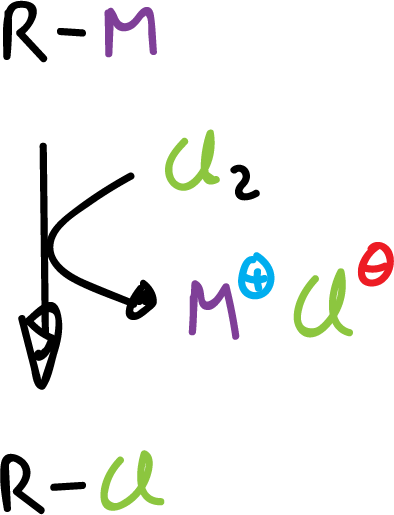
This can be considered as an electrophilic substitution reaction.
Reaction with oxygen
Organic compounds of many metals react rapidly with oxygen. Some are so reactive that they ignite instantly on contact with air.
Alkyl boranes burn with a bright green flame. Because of this reactivity, it is common to carry out all organometallic reactions in an inert atmosphere. For example, using nitrogen or argon.
Reacción con otros compuestos organometálicos
There are numerous reactions of one organometallic compound with another. For example, alkyl-copper compounds react with alkyl-lithium compounds to give products known as cuprates. These cuprates are very useful as reagents in synthesis.
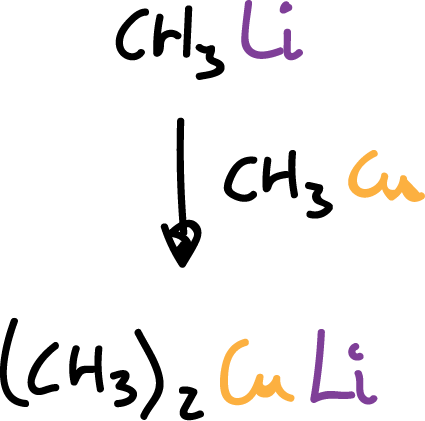
Cuprates, when in solution, exist in a dimeric or tetrameric form.
Uses of organometallic compounds
They are very remarkable reagents for the synthesis of other organic compounds. The most widely used in this respect are the Grignard reagents.
Some of the most commonly used reactions of Grignard reagents are summarized in the following scheme.
fig-19
Alkyl lithium compounds also perform most of these reactions and are used in the same things.
Alkylboranes are equally relevant and hydroboration is a widely used method for the preparation of alcohols.
fig-20
Also, alkyl mercury compounds are very useful as intermediates in the formation of alcohols and ethers.
fig-21
Organic cadmium compounds and cuprates are often used to prepare ketones from acyl halides.
fig-22
Solved problems on organometallic compounds