Written by J.A Dobado | Last Updated on April 22, 2024
What is laboratory safety?
The work in the organic chemistry laboratory involves a series of risks inherent to its nature due, on the one hand, to the chemical products and solvents that are handled, and on the other hand, as a consequence of the techniques used in the development of the experiments. Although laboratory practices try to minimize these risks, there is always the possibility of an accident of greater or lesser severity. For example, cuts, burns, splashes, spills, or even in the worst case, fires and explosions. Therefore, it is necessary for the student to acquire safe working habits from the beginning, and to know the basic safety measures and regulations that exist for this purpose, being able to identify the potential sources of accidents, in order to be able to act correctly in case they occur, and minimize the consequences of such accidents.
The most relevant aspects of laboratory safety are described in a general way; however, there are specialized monographs in the literature that deal with all aspects related to laboratory safety in a more extensive way.
Making the laboratory a safe place
Before starting work in a laboratory, we must make sure we know the location of the different emergency equipment in case they are needed in situations where the speed of intervention is critical. We should know where to go in case of an accident and know perfectly the evacuation zones of both the laboratory and the building. In addition, we should know where to call in case of an accident and have the emergency telephone numbers of the fire department, medical emergencies and the National Institute of Toxicology visible.
Safe behavior and habits
The student should learn good practices to be able to work safely with chemicals and materials that may be hazardous, avoiding, in any case, careless or negligent behavior. This entails taking the appropriate precautions at all times and asking the teacher any questions regarding handling and how to act.
The risk of accidents can be minimized as follows:
- Consult safety data sheets (MSDS) and labeling for all reagents to be handled in a laboratory experiment or practice, knowing the potential hazards associated with the handling, as well as how to neutralize or diminish the possible harmful effects in case of an accident.
- Study and document, prior to performing the experiment in the laboratory, the techniques and procedures (it is necessary to know in detail the reason for all the basic operations and manipulations that will be carried out).
- Follow all safety instructions specific to each experiment to be performed in the laboratory.
- Know the location and correct operation of all general emergency equipment in the laboratory.
- Keep our workplace clean and tidy at all times, to avoid the consequences of possible spills, broken glassware, etc.
- Before using a particular compound, make sure that it is the desired one. If necessary, read the label twice.
- Never return residues of used products to the original bottles without consulting the teacher.
- Any new solution prepared should be stored in a clean and suitably labeled container.
- Do not touch chemicals with your hands, much less with your mouth. Do not pipette with your mouth either, use a rubber bulb, pipette controler or dispenser.
- When you want to dilute an acid, such as sulfuric acid, always proceed by adding the sulfuric acid to the water. Never pour water over acid as this may cause splashing or strong heat release.
- Do not smell directly any chemical product, as it may be irritating, tearing, harmful, etc.
- Keep flammable solvents away from heat sources such as stoves, ranges, oil baths, etc.
- When handling glass material, be extremely careful with sharp edges and tips. Always keep them away from eyes and mouth.
- Protect hands with gloves or rags when inserting a stopper into a glass tube.
- Hot glass is not distinguishable at first glance from cold glass. If a glassware is inside a stove or in contact with a heat source, in order to avoid burns, it is necessary to use tongs or other protection system to handle it and let it cool down before touching it directly or manipulating.
- Never heat a closed system, as this may cause an explosion.
- Each time glassware is assembled, check the assembly and clamping before starting the experiment.
- Do not eat, drink or chew gum in the laboratories.
- Always pay attention to the work being done and maintain a responsible attitude.
Causes of accidents in the laboratory
The main cause of accidents in an organic chemistry laboratory has its origin in an incorrect handling of dangerous chemical substances, laboratory material and equipment. Therefore, prior to the realization of any practice, it should be thoroughly prepared with the consequent careful use of compounds and laboratory equipment. In this way we will reduce, as far as possible, the probability of such accidents occurring.
Among the most frequent causes of accidents in the laboratory, the following cases can be considered:
- Lack of order and cleanliness in the workplace.
- Inadequate use of personal protective equipment and of common use.
- Negligent use of glassware.
- Inadequate transfer of liquids in general.
- Distillations in open collector near a flame or heat source.
- Hot plates at high temperatures and/or other heat sources.
- Purification of solvents that may contain peroxides (e.g. ethers).
- Spontaneous ignition of catalyst residues or Zn residues from reductions.
- Oil baths heated to temperatures above 160 °C.
- Negligent destruction of Na, K, NaNH2, LiAlH4, CaH2 residues.
- Vacuuming in Erlenmeyers or other types of containers not prepared for this purpose.
- Not following the teacher’s instructions at all times.
What to do in case of an accident?
Although not exhaustive, the following are some recommendations to try to minimize the consequences of a possible accident:
- Accident (in general). Immediately notify the teacher or person in charge of the laboratory. In case of seriousness call emergency number. Warn people close to the area about the nature of the emergency. Do not move any injured person, except in case of fire hazard or exposure to chemicals.
- Accidental ingestion of chemical products. It is necessary to contact immediately the Toxicological Information Service and go to the emergency department with the label and the product. If the patient is unconscious, lay him/her down with the head on his/her side. Do not give him/her to ingest any liquid, nor provoke vomiting.
- Chemical inhalation. Immediately evacuate the affected person to a place where he/she can breathe fresh air, go immediately to the nearest emergency health service.
- Fire in the laboratory. Evacuation according to the indications of the teacher or person in charge. If the fire is small and localized, an attempt should be made to extinguish it with a fire blanket or fire extinguisher. Attempts should be made to remove flammable chemicals near the fire. Water should never be used to extinguish a fire caused by organic solvents.
- Body fire. If your clothes catch fire, call for help immediately, lie down on the floor, rolling on your back to try to put it out. Never run or try to reach a safety shower unless it is very close. We must help someone who is burning using a fire blanket.
- Burns. Small burns can be treated by washing the affected area with cold water. In case of more serious burns, immediately go to the nearest emergency health service.
- Cuts. They must be washed well, with plenty of running water. If they are small and stop bleeding in a short time, apply an antiseptic and cover with suitable dressings. If they are large and do not stop bleeding, go immediately to the nearest emergency health service.
- Chemical splashes on the skin. Wash immediately with plenty of running water. Use showers and eyewash in cases where the affected area of the body is large and washing in a sink is not sufficient. Rapid washing is very important to reduce damage. In case of contact with the eyes of corrosive products, the time of action is essential (less than 10 s). Medical assistance is necessary, no matter how small the injury may seem.
- Chemical spills. In case of spills of liquid products, act quickly to neutralize, absorb and eliminate them. Evacuate the laboratory, if necessary, and use the corresponding protective material. Absorb the spilled liquid with a material as inert as possible (vermiculite, sand, etc.). If the liquid is flammable, all possible sources of ignition must be cut off from the area and it must be absorbed with activated carbon or other specific absorbents. Sawdust should never be used due to its flammability. Strong acid spills should be quickly adsorbed using neutralizing absorbents. Alternatively, sodium bicarbonate can be used for neutralization. In the case of spills of strong bases, specific commercial products should be used, or else abundant water at a slightly acid pH should be used.
Personal protective equipment
Personal protective equipment in an organic chemistry laboratory should consist of the following items: safety glasses, lab coat, gloves and in some cases a face mask (see Figure).
Regarding the type of clothing that should be worn while performing practical work in the laboratory, the use of shorts, sandals or any other type of uncovered footwear is especially discouraged. It is advisable to wear your hair tied back and avoid wearing pendants, scarves, bracelets or clothes with wide sleeves that could get caught in assemblies, apparatus, furniture, etc. or come into contact with flames or heat sources.
Safety goggles
Safety goggles protect our eyes from splashes, so they should be worn at all times, whenever one is in the Organic Chemistry laboratory. Contact lenses should not be worn, since the effect of

chemical products in the eyes is enhanced if these products are introduced between the contact lens and the cornea. On the other hand, prescription glasses do not guarantee adequate protection, so safety glasses should be worn over them.
In the case of organic contact lenses, exposure to solvent vapors or splashes causes a significant deterioration on this type of material. This is not the case with safety goggles, as they are specially manufactured for these purposes. In addition, safety goggles must be designed to provide good front and side protection. They should be as comfortable as possible, adjusting to the nose and face, and should not interfere with the user’s movements.
Lab coat
The lab coat is a safety item that protects the body and clothing from chemical splashes. They are usually made of cotton or natural fibers, since synthetic fibers do not resist corrosive substances. Cotton or natural fibers, when burned, do not stick to the body, so if a corrosive substance falls on it, it will be easily removed. The gown should always be buttoned and cover up to below the knee.
Gloves
Protective gloves should be used especially when handling corrosive chemicals with potential health hazards. Depending on the type of glove, specific precautions must be taken since no material offers permanent protection against all chemicals.
Before using them (especially latex gloves), make sure that they are in good condition and do not have holes, punctures or tears. If the gloves are leaking reagents, they should be disposed of in the appropriate waste container.
Before use (especially latex gloves), make sure that they are in good condition and have no holes, punctures or tears. If gloves leak reagents, they should be disposed of in the appropriate waste container.
- Plastic. Protects against mild corrosive substances and irritants.
- Latex. Provides light protection against irritants (some people may have an allergic reaction to latex).
- Natural rubber. Protects against mild corrosive substances and electric shocks.
- Neoprene. For working with solvents, oils, or slightly corrosive substances.
- Cotton. Absorbs perspiration, keeps objects being handled clean, fire retardant.
- Zetex gloves. For handling small, very hot objects.
When working with extremely corrosive materials (e.g. HF), it is necessary to wear thick gloves.
Gloves should be removed before leaving the laboratory to avoid contamination, and also when handling commonly used objects, such as telephones, notebooks, pens, computers, etc. In addition, care should be taken when removing them. The correct way to do this is to pull from the wrist towards the fingers, making sure that the outside of the glove does not touch the skin. Disposable gloves should be disposed of in the designated containers.
Face masks

These are personal safety elements for the protection of the respiratory tract against particles, vapors and gases. Depending on the level of safety required, there is a wide range of models on the market. The most common are the following:
- Self-filtering respirators: They act as a barrier against particles and dust. They are the easiest to use and the most widespread. For example, in the case of transferring silica gel or alumina, it is necessary to use this type of mask or perform the operation in a fume cupboard.
- Filtering equipment: They consist of a filter and a facial adapter. The filter is designed to act as a barrier against particles (mechanical filters), against vapors and/or gases (chemical filters), or a combination of both (mixed filters), while the facial adapter can be a mask, mask or mouthpiece.
Emergency equipment for general use
These are safety elements, of common use, that should be present in every laboratory, since they allow us to minimize the risks in case of accidents. We must know their location and operation, so that in case of emergency we can act efficiently (see Figure).
Safety shower
It is commonly used in cases of projections
of chemicals with a risk of chemical burns, and even if clothing catches fire. It must provide sufficient water flow to wet a person quickly and have an easy to use opening system.
Eye wash
The eye wash is intended to clean the eyes of a person after an accident in which contaminated materials or foreign substances may have penetrated. It allows quick and efficient cleaning of the eyes and face. It is composed of two opposing taps, each of which projects a jet of water at low pressure so as not to cause pain directly to the eyes. The water is then collected in a fountain with a sump designed for this purpose.
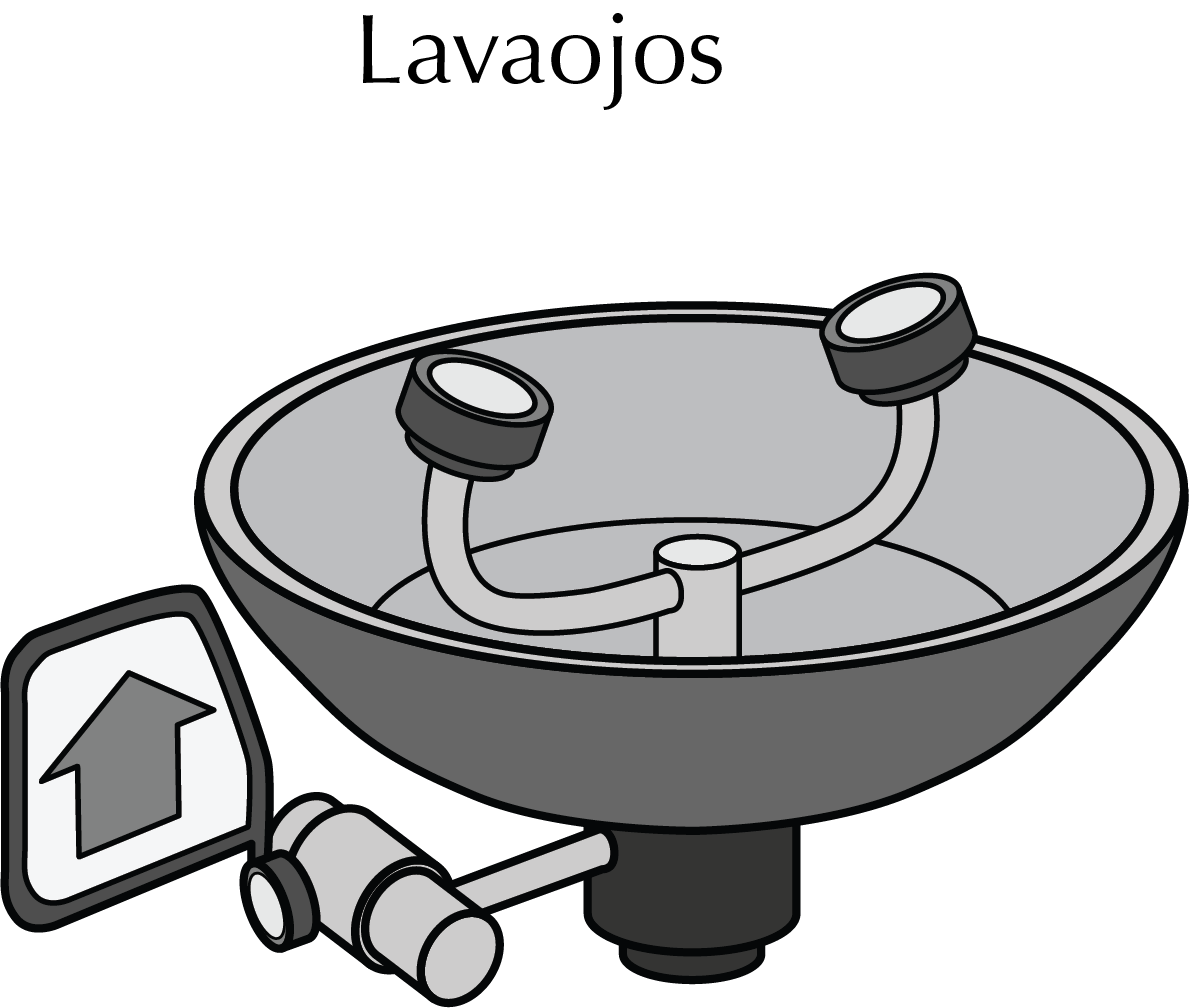
It is essential to know perfectly the location and operation of this emergency device because in case of an accident we must be able to use it as quickly as possible in situations where vision may be partially or totally limited. For a correct use, the eyes should be kept open with the help of the fingers to facilitate a correct washing under the eyelids.
Fireproof blankets
Fire blankets are designed to extinguish small fires. They consist of a sheet of fireproof material that must be placed over the fire to extinguish it, as it prevents the supply of oxygen to the fire.
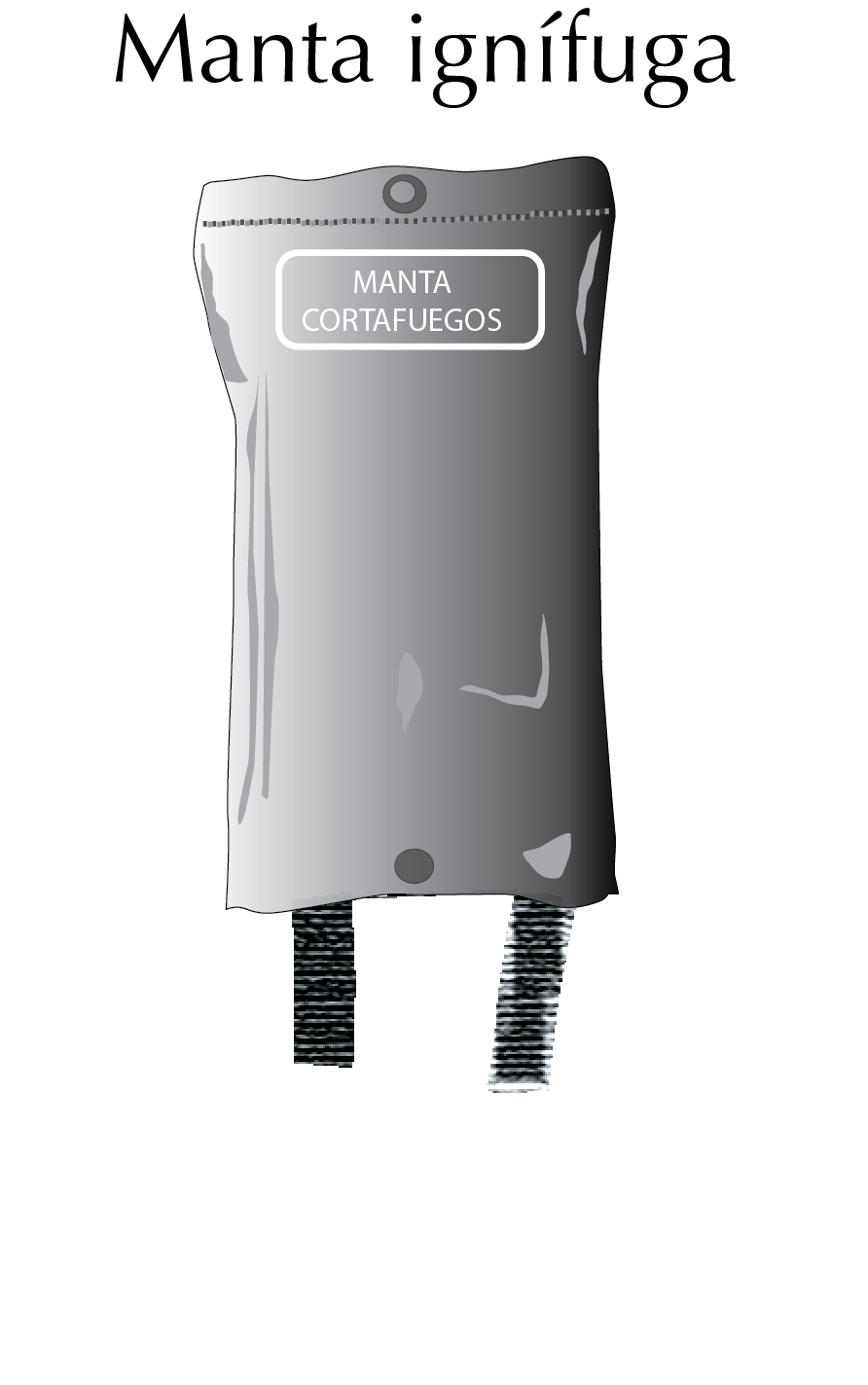
Fire blankets should be deployed quickly, covering the entire object or area on fire, taking care not to burn our hands while deploying them (fold the blanket around our hands beforehand).
If this type of device is used quickly and correctly, we will be able to reduce fires without the need to use fire extinguishers, since the latter cause a lot of collateral damage to electrical material and equipment by spraying jets of foam under pressure in the laboratory.
They are usually specially folded in a sheath for quick release and are made of flame retardant fibers such as Nomex® or fiberglass and sometimes impregnated with a flame retardant.
Fire extinguishers
They are metal containers in the form of a cylinder or steel cylinders containing a pressurized fire extinguishing agent. When we open a valve, the agent comes out through a nozzle that is directed to the base of the fire
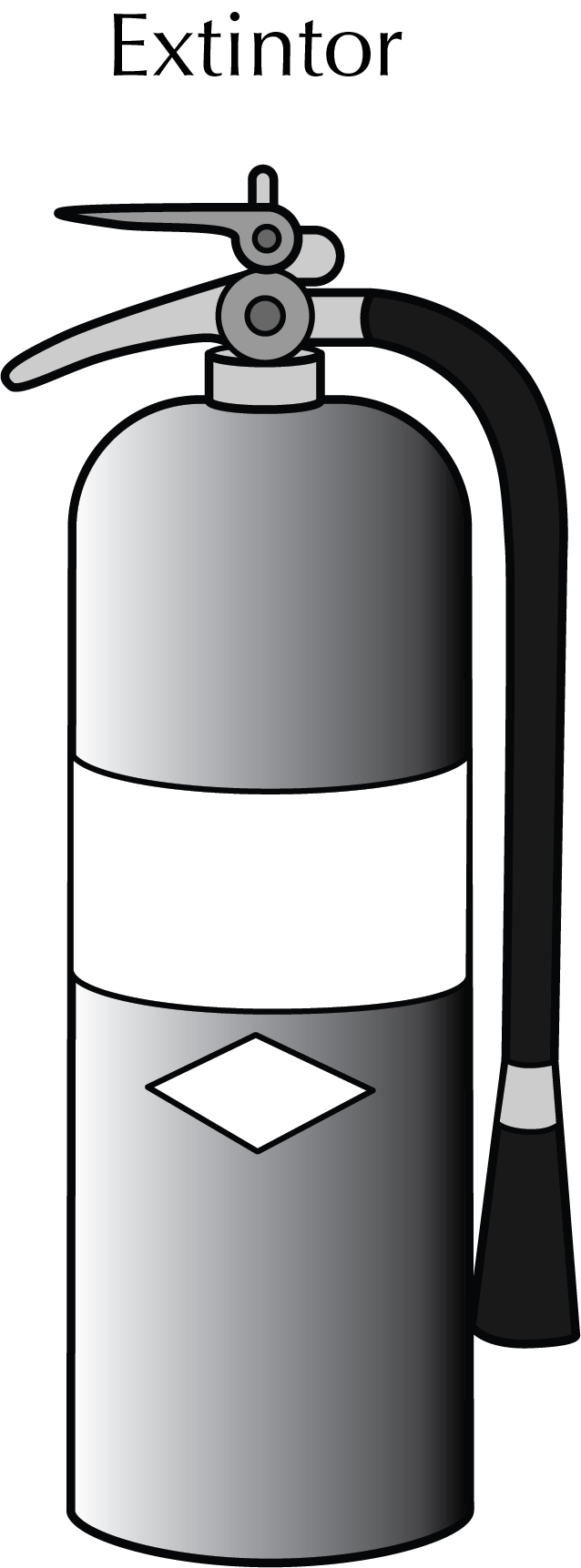
(it should never be directed to the upper part of the flame). They usually have a seal to prevent accidental activation of the extinguisher. Before use, it is important to give a sharp blow with the base of the extinguisher on the floor, so that the content is homogeneous.
When small fires in the laboratory cannot be controlled with fire blankets, fire extinguishers should be used quickly. But be aware that there are different types of extinguishing agents suitable for different classes of fire. The use of an unsuitable type of extinguishing agent for a particular type of fire can be counterproductive. Therefore, it is necessary to know in each case of fire the appropriate type of extinguisher.
Fire extinguishing agents
These are substances of special composition, contained in fire extinguishers, which are used to extinguish fire.
- Class “H” extinguisher, halogenated. They are only acuthorized in some military applications because their composition destroys the ozone layer. Recommended in closed environments without the presence of life. Contains a suffocating agent that consumes the oxygen present.
- Class “N” extinguisher. Neutralizers to the formation of gases by chemical agents or weapons of mass destruction. They are composed of powder of the corresponding neutralizing agent.
- Water spray. They are designed to protect all areas containing Class A fire hazards (solid fuels) efficiently and safely.
- Demineralized water. Suitable for class C fires (flammable gases) connected equipment, or chemical fires or bacteriological risks.
- Water and Foam (AFFF). Designed to protect areas containing Class A (solid fuels) and Class B (liquid and gaseous fuels) fire hazards.
- Carbon dioxide (CO2). Designed to protect areas containing Class B (liquid fuels) and Class C (flammable gases) fire hazards.
- All-purpose chemical powder (ABC). The dry chemical powder (75 % mono ammonium phosphate) is used for firefighting class A (solid fuels), class B (liquid fuels), class C (flammable gases).
- Dry Chemical Powder (BC). Designed to protect areas containing Class B (liquid fuels) and Class C (flammable gases) fire hazards.
- Chemical dust (D). Designed to protect areas containing class D fire hazards (combustible metals) including lithium, sodium, sodium and potassium alloys, magnesium and metal compounds. It is charged with sodium borate based composite powder.
The most commonly used extinguishers in the laboratory are polyvalent chemical powder extinguishers. In addition, special powder or dry sand should be used to extinguish metallic fires.
Chemical absorbent
In case of spills of hazardous liquid substances, absorbent solids are usually used, which are manufactured with a special formulation (high active adsorption surface) which allows to quickly control the spilled liquids, preventing the spill from reaching a large surface area. The use of sawdust as absorbent material is strongly discouraged due to its ease of combustion. The most common absorbents are used in the form of granules of different sizes and can be classified as follows:
- Oxygen compounds of hydrophilic and polar nature (silica gel or zeolites). Silica gel is often used in drying liquids or gases, and also for adsorption of high molecular weight hydrocarbons in natural gas. Zeolites are also used in gas drying and removal of CO2 from natural gas.
- Carbon compounds of hydrophobic and non-polar nature (activated carbon and graphite). Activated carbon is used for adsorption of organic molecules and non-polar compounds and also for gas and water purification.
- Polymeric materials that present polar or non-polar functional groups in a porous polymeric matrix.
Safety partition
It is a portable panel that are generally made of polycarbonate and offer protection against splashes and projections of reagents and against small explosions/implosions of glass material, etc. They are usually used, for example, in rotary evaporators, in flash chromatography, etc.
Fume hood
Enclosure where an experiment can be performed safely since they have an aspiration system that eliminates hazardous, dangerous or smelly vapors and gases that may originate when performing chemical reactions or handling toxic substances. They have a safety glass screen that protects us from accidents such as splashes of liquids or glass material that may break, small explosions, etc. (see Figure). All the basic operations that require it must be carried out inside the fume hood, since they have the services of a laboratory bench, such as water or vacuum circuit, gas and electric current connection or devices for holding the laboratory material and assemblies.
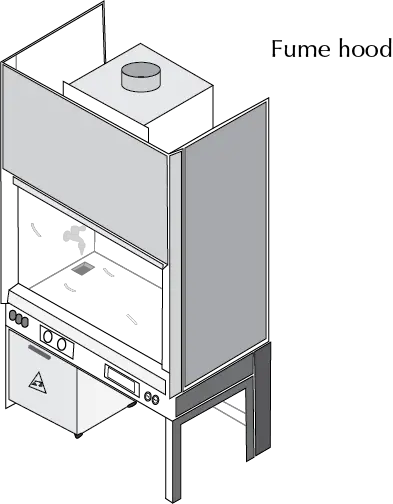
The fume cupboards allow safe working in the laboratory because:
- They collect emissions generated by hazardous chemicals.
- They protect us against splashes and projections.
- They allow us to work in an area of the laboratory that is safe from ignition sources.
- Depending on the design, they protect against small explosions.
- They facilitate air renewal in the laboratory.
- They create a low-pressure area in the laboratory that prevents contaminants from escaping to adjacent areas.
- In addition, they must allow the visualization of the development of the experiment.
Recommendations for a correct use of the fume hood:
- Do not use it as a storage facility for hazardous reagents.
- The interior of the fume hood should remain as clear and clean as possible at all times.
- Avoid the generation of polluting gases at high velocities, so that they can be properly aspirated, handling the minimum amounts of reagents.
- The fume hoods should be opened to the minimum that allows us to work properly and that no harmful gases can escape. Never work with your head inside these enclosures.
- At the end of the experiment, leave the fume hoods operating with the windows closed so that the gases and vapors generated are completely eliminated.
Glove box
It is a sealed cabinet that is designed to allow handling of reagents and objects in such a way that they are always away from the operator. It features gloves arranged in such a way that the operator can place his hands and arms inside these and manipulate inside the box without breaking the insulation. The boxes are usually transparent to allow a better view of what is being handled (see Figure).
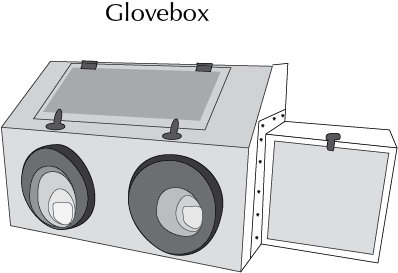
Dependiendo del uso se clasifican en dos tipos:
- Handling of hazardous substances (radioactive or infectious disease agents, etc.).
- Handling of substances and reactions that must remain in an inert, sterile, dry, dust-free or very high purity atmosphere.
Fireproof cabinet
They are cabinets made of insulating and non-combustible materials that protect the products stored inside against fire. The level of protection is labeled with the initials RF (fire resistance) followed by a number indicating the number of minutes that can elapse since the fire occurs without the interior temperature exceeds 180 ºC. Examples of resistance levels are RF15, RF30, RF60, RF90 or RF120. They usually have other protection systems such as expansion joints which, when heated to over 50 ºC, expand to seal cracks in doors and ventilation ducts, protecting the stored products from an external fire or smothering an internal fire. These cabinets are used for the storage of flammable, explosive, toxic, corrosive, solvents, etc. products.
Cold chamber
The use of domestic refrigerators is not recommended for the storage of volatile and flammable organic solvents, especially in large quantities. For this purpose, cold chambers should be used, which are refrigerators or rooms with sufficient capacity for the storage of solvents or chemical products at low temperatures. They are specially designed to protect against explosions and fires caused by flammable vapors and are therefore equipped with air renewal systems. They are designed so that the electrical components are outside the storage enclosure and the compressor must be sealed and located in the upper part of the cold chamber.
Safety containers
- Safety containers: when volatile and flammable organic solvents need to be transported or stored, containers specially designed for this purpose must be used. They are made of metal (reinforced steel and raised bottom for better resistance to impacts) with an automatic closure system of the cap with a spring-loaded spring that avoids possible leaks. In addition, this closure system works as an overpressure valve and has a double flame-retardant filter, so that even if it burns the vapors do not reach the stored products.
- Dewar container: is a container designed to provide thermal insulation, and are used to store hot or cold liquids. They are constructed with a double wall of silvered glass (reflects radiation) and have a vacuum inside the container (prevents heat loss by convection and conduction). They can also be made of stainless steel, which has the advantage of being more resistant and better able to withstand temperature changes. They are usually used to store liquid nitrogen (e.g. -196 ºC), liquid oxygen (e.g. -183 ºC), etc.
Viedo about Lab Safety Standards
FAQ
What is laboratory safety?
Safety measures in laboratories are a set of preventive measures aimed at protecting the health of those who work there against the risks arising from the activity, to avoid accidents and contamination both within their work environment, as well as to the outside.
What is forbidden to do in the laboratory?
It is absolutely forbidden to work alone in a laboratory, to eat, drink or smoke inside the laboratory and to block doors or access ways. When leaving the laboratory, hands must be washed.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2