Written by J.A Dobado | Last Updated on April 22, 2024
What is vacuum distillation?
Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºC, or with a lower boiling point but which are thermally unstable. Vacuum pumps can be used as a vacuum source.
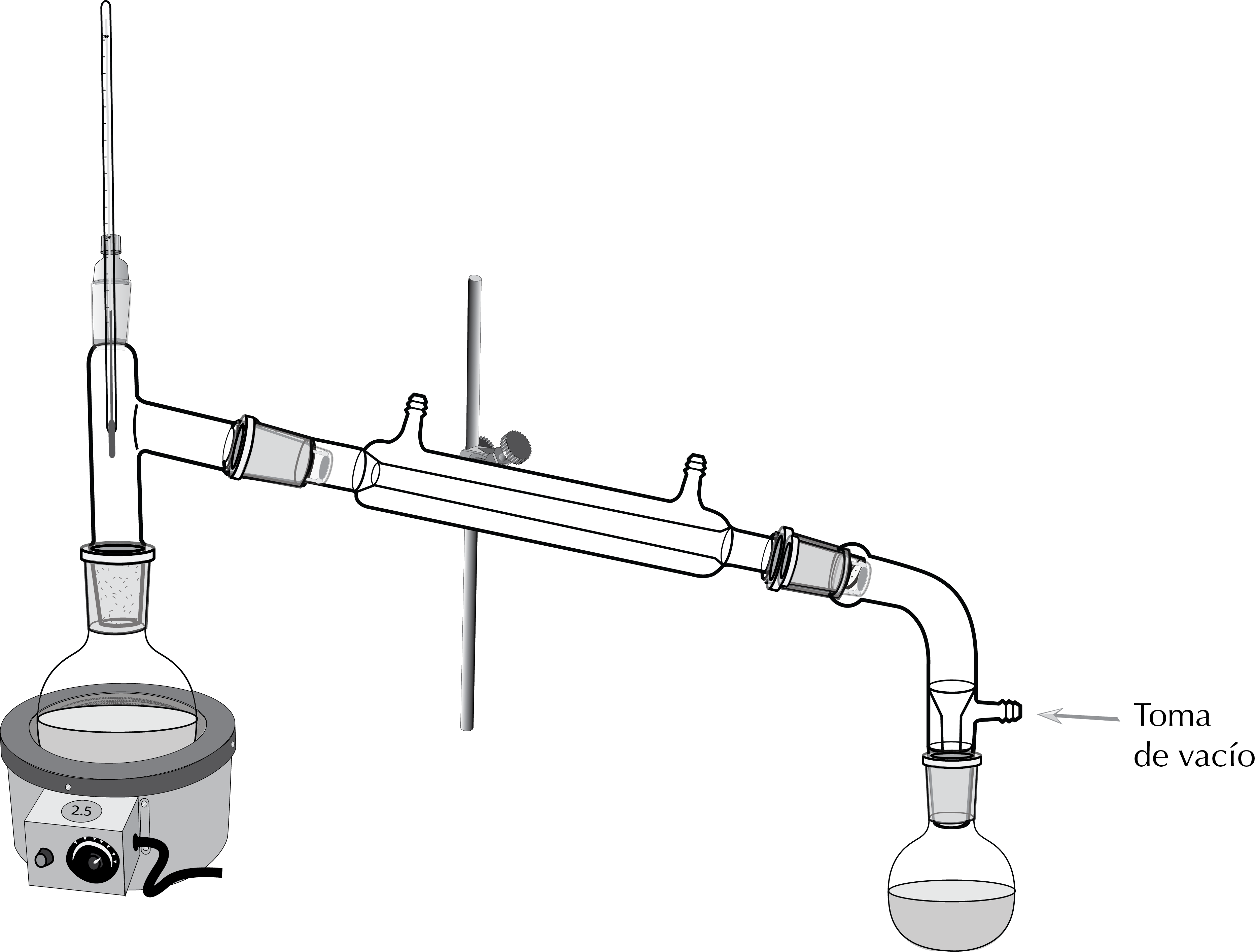
Mounting schematic diagram
Vacuum distillation devices can have different configurations and components, but the most popular one consists of an assembly very similar to simple distillation, except that it has a distillation tail connected to vacuum.
Warnings
- The glassware used in a vacuum distillation must be carefully examined for scratches or cracks as this poses a risk of implosion of the assembly. Especially in the case of very low pressures, the assembly can be protected by wrapping it in some kind of mesh or with adhesive tape, so that in case of an accident, glass fragments will not be dispersed.
- The ground joints must be carefully greased to ensure the best fit and to avoid vacuum losses.
Vacuum distillation assembly
The set-up for vacuum distillation is similar to that of simple distillation, but with some differences. For example, a special receiving adapter with a vacuum connection is used.
On the other hand, boiling chips are not usually used for homogeneous boiling, since the air occluded in the porous plate can be quickly released when the vacuum is created, and the vacuum loses its effectiveness. Alternatively, magnetic stirring is used.
Sometimes, as when the amount of liquid to be distilled is small, a special setup (Claisen) for vacuum can be used which requires the use of a capillary as a stirring and vacuum regulation system.
In the case of a single compound, proceed as indicated:
- First, the clamp is attached with the clamp holders to the metal rod of the ring stand or fume hood.
- The ground joints of all assembly components are greased with silicone grease.
- The round bottom flask is then placed on the hot plate and its mouth is adjusted with a clamp.
- The liquid to be distilled is added (stir bar for magnetic stirring).
- Then, mount the distillation head on the round bottom flask.
- For liquids with a tendency to foam or splash, it is convenient to insert a Claisen adapter between the flask and the water cooler condenser.
- Subsequently, the thermometer with its adapter is placed on the top of the three-way adapter.
- We attach the water-cooler condenser with a clamp and clamp holder to the metal rod and with a clip to the three-way adapter.
- We also connect the water-cooler condenser to two pieces of Tygon tubing (the lower connection goes to the water source, and the upper to the drain).
- The receiving adapter is mounted and a receiving flask is attached to it.
- If necessary, the collecting flask is placed in an ice bath to facilitate condensation of the distillate.
- Connect vacuum line.
- We open the vacuum pump pressure gauge.
- In some cases it is appropriate to cover the flask and the distillation head (or Claisen adapter) with aluminum foil to avoid heat loss.
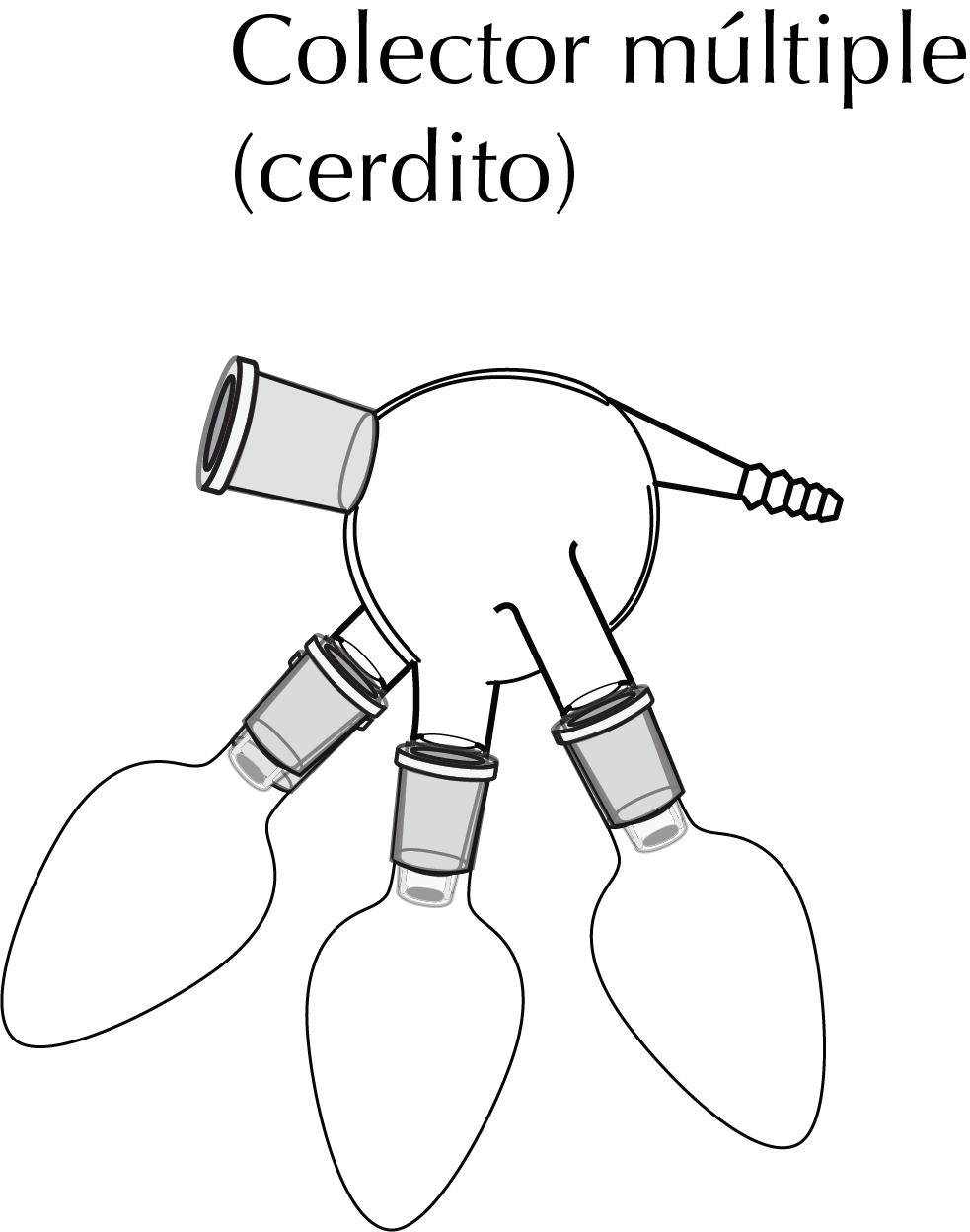
To distill a mixture with two or more components, a special distillation glue is used which is called a multiple collector. It usually features two or three male-type fittings, to which a round bottom flask (with ground joint) is connected to each. By turning this adapter, the different fractions are collected.
Principle of vacuum distillation
Boiling of a liquid or solution begins when its vapor pressure is equal to the external or applied pressure (usually atmospheric pressure, 760 mmHg). Therefore, if we reduce the value of the external pressure, the boiling point of the liquid also decreases, as shown in Figure.
This is because a lower vapor pressure is necessary for the liquid to boil, since it is carried out at a lower temperature.
Examples
For example, water produces a vapor pressure of 760 mmHg at 100 ºC, as shown in Figure. Therefore, water boils at sea level at 100 ºC.
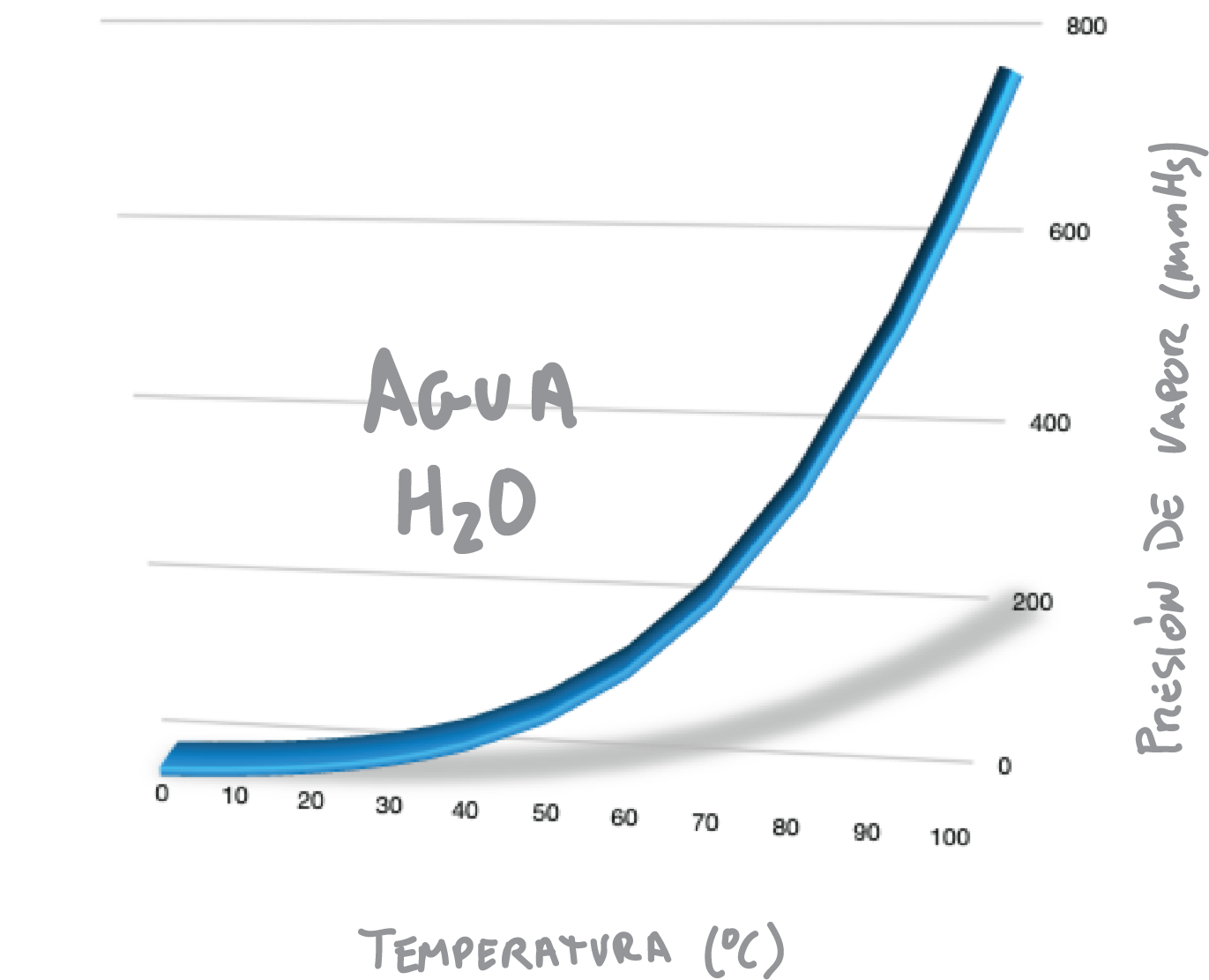
However, when water is subjected to a vacuum of only 100 mmHg it would boil at approximately 50 °C, since this is the temperature that produces a vapor pressure of 100 mmHg (see Figure). This behavior is explained by the Clausius-Clapeyron equation.
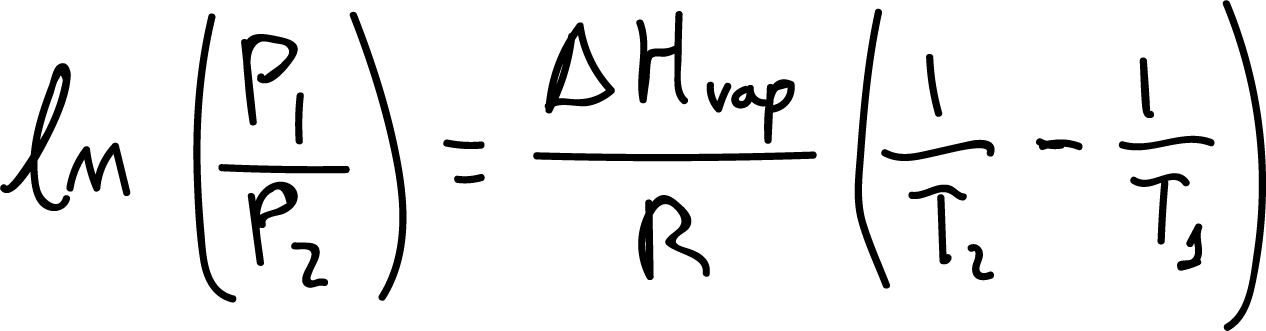
Where, P1 and P2 are the vapor pressures at temperatures T1 and T2 (in degrees Kelvin), respectively. ΔHvap is the enthalpy of vaporization of the substance, and R is a constant equal to 8314 J /mol-K.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2