Go to the page with the solutions to the problems.
Amines – list of problems
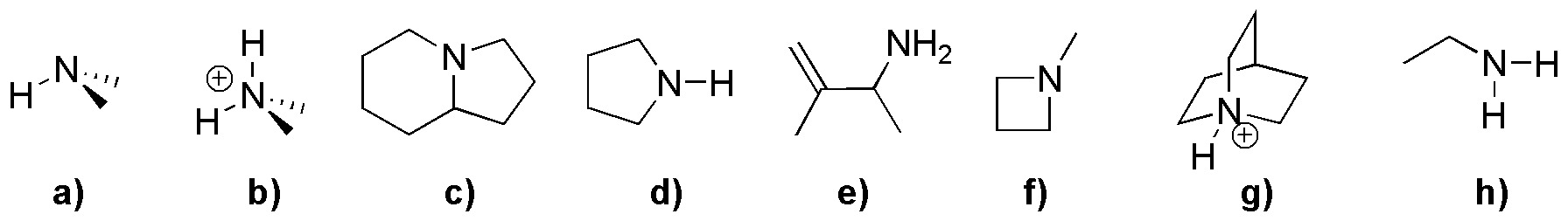
Problem 1)
Classify the following compounds (a-h) as primary amines, secondary amines, tertiary amines and quaternary ammonium salts, as appropriate.
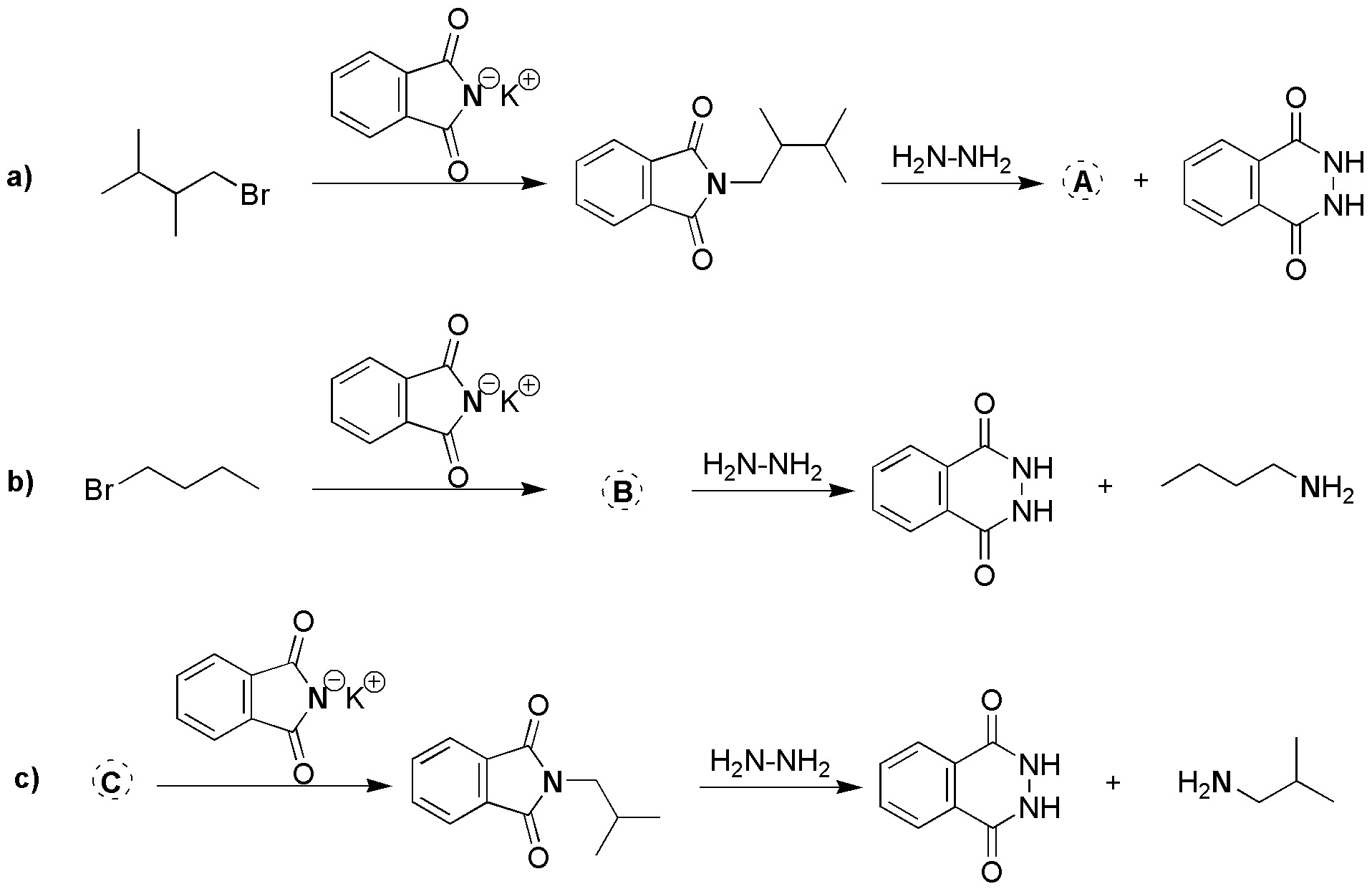
Problem 2)
Complete the following Gabriel syntheses (a-c).
Problem 3)
Order in increasing order of basicity the following compounds, justifying the results.

Problem 4)
a) Order from higher to lower basicity the following amines, justifying the result.
- (I) Methylamine
- (II) Aniline
- (III) p-Chloroaniline
- (IV) Trimethylamine
- (V) Tetramethylammonium bromide
b) Which amine in the series will be most effective as a nucleophile?
Problem 5)
Describe a simple method to obtain N-methylbenzamide from methylamine.
Problem 6)
When pentyl amine is treated with nitrous acid in the presence of hydrochloric acid, nitrogen is observed to be given off and a mixture of 5 products is formed. Justify the result.
Problem 7)
An amine of molecular formula C6H15N reacts with one mole of CH3I followed by hot wet silver oxide to give but-1-ene. Deduce which amine it is.
Problem 8)
An aqueous solution of sodium hydroxide and benzenesulfonyl chloride is reacted with an amine of molecular formula C4H11N to give a precipitate. From this result deduce the structure of the amine.
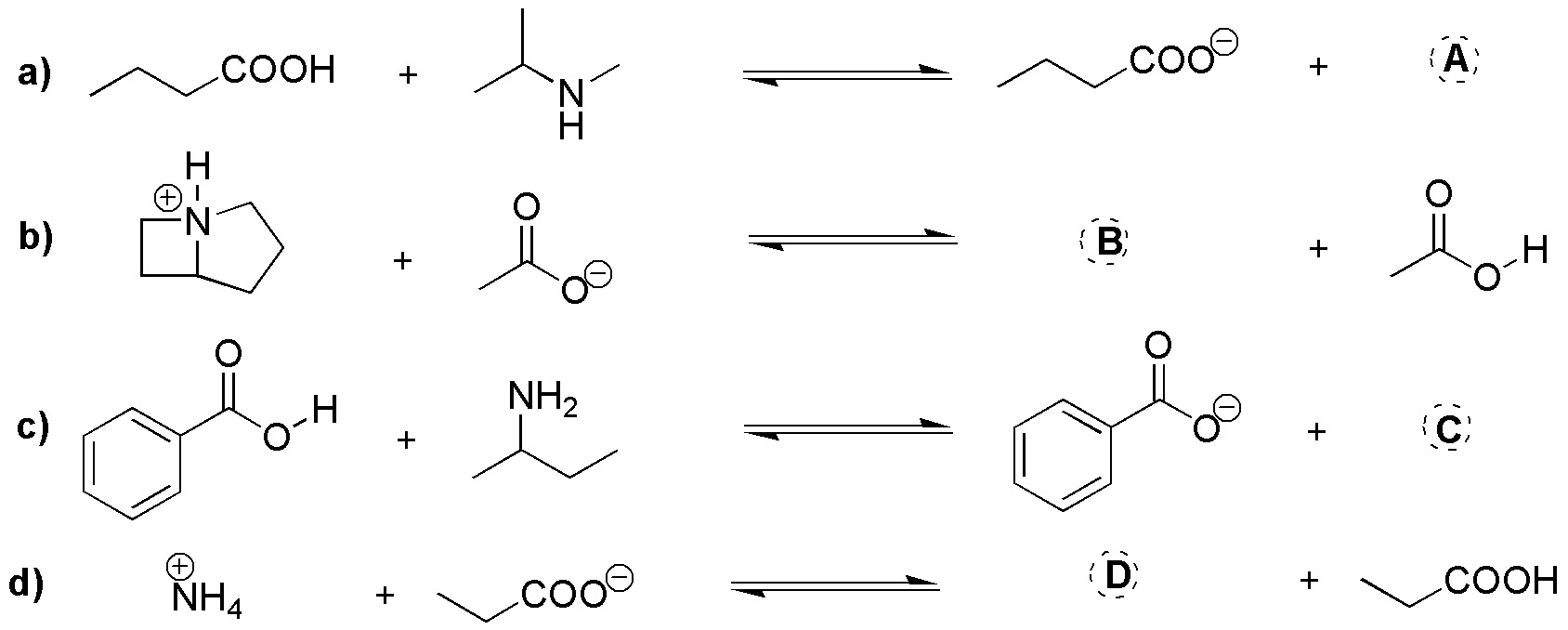
Problem 9)
Complete the following reactions (a-d):
Problem 10)
Normally, the alkylation of a primary or secondary amine does not stop at monoalkylation because the product reacts back with the excess of the amine used as a reactant. Write all the products for the alkylation reaction between ethylamine and methyl iodide.
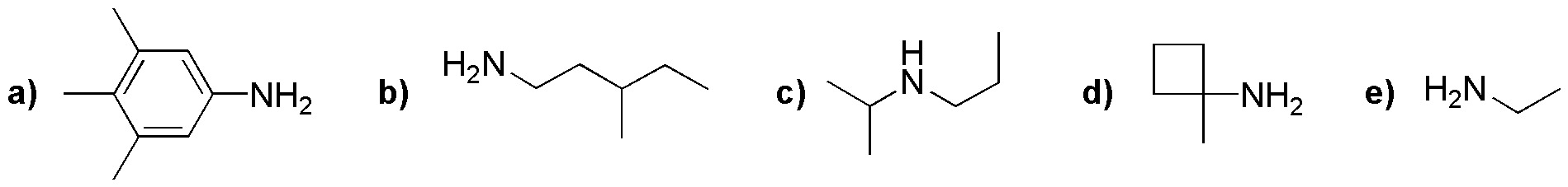
Problem 11)
Justify whether amines (a-f) can be obtained by a Gabriel synthesis.
Problem 12)
What reagents (a-f) are needed to obtain the following compounds (I-III) by the Mannich reaction?
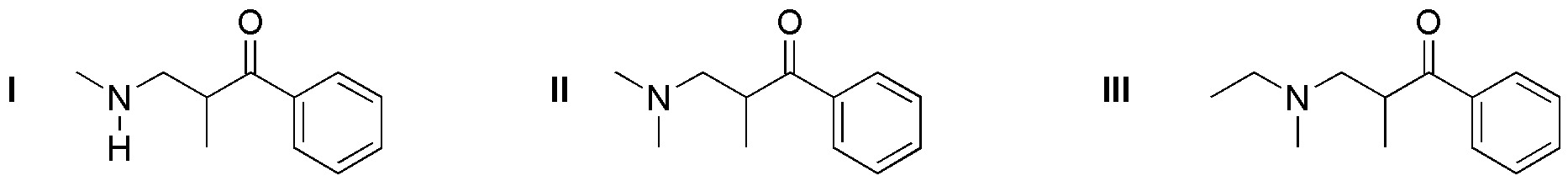
- a) CH3CH2NHCH3 + CH2O + HCOPh
- b) (CH3)3N + CH3CH2COPh
- c) CH3CH2NHCH3 + CH2O + CH3CH2COPh
- d) CH3CH2CH2N(CH3CH2)CH3 + HCOPh
- e) CH3NH2 + (CH3)2CHCOPh
- f) CH3CH2N(CH3)2 + CH2O + HCOPh
- g) (CH3)2NH + (CH3)2CHCOPh
- h) CH3CH2N(CH3)2 + CH3CH2COPh
- i) (CH3)2NH + CH3CH2COPh
- j) CH3CH2N(CH3)2 + CH2O + CH3COPh
- k) CH3CH2CH2N(CH3)2 + HCOPh
- l) (CH3)2NH + CH2O+ CH3COPh
- m) CH3NH2 + CH2O + CH3CH2COPh
- n) (CH3)3N + CH2O + CH3COPh
- o) (CH3CH2)2NCH3 + CH2O + HCOPh
- p) (CH3)2NH + CH2O+ CH3CH2COPh
- q) CH3CH2NHCH3 + (CH3)2CHCOPh
- r) CH3CH2CH2NHCH3 + HCOPh
Problem 13)
Write which intermediate and major product is obtained in the following sequences of reactions ending with Hofmann elimination.
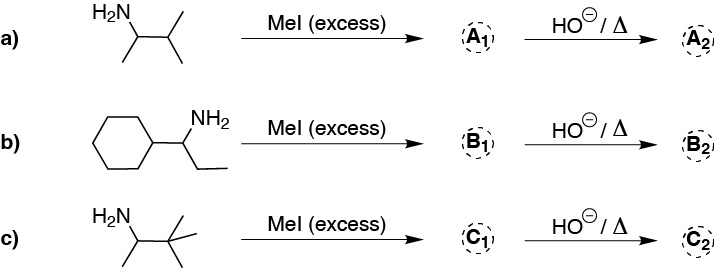
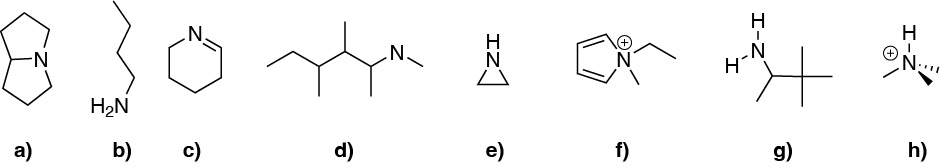
Problem 14)
Classify the following compounds (a-h) as primary amines, secondary amines, tertiary amines and quaternary aminium salts.
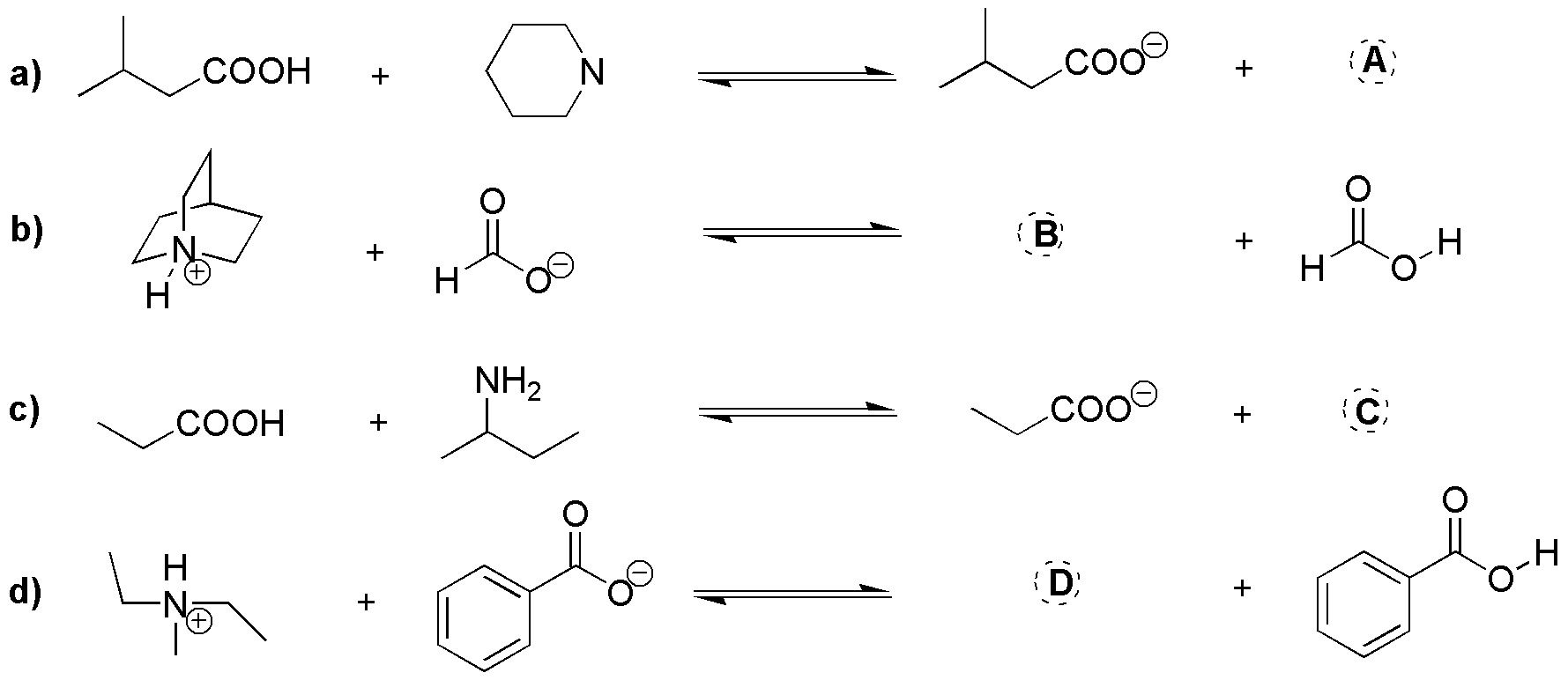
Problem 15)
Complete the following acid-base reactions of amines (a-d).
Problem 16)
Write the major product obtained in the second Hofmann elimination steps.
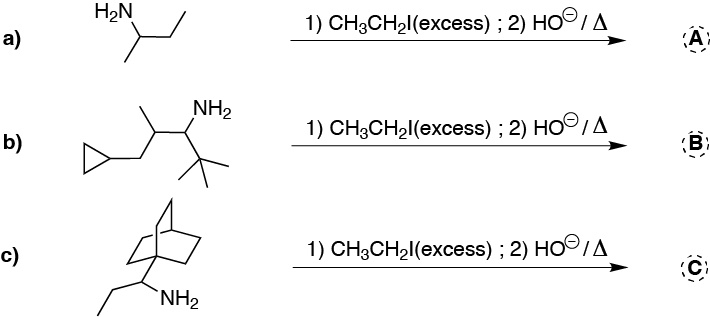
Problem 17)
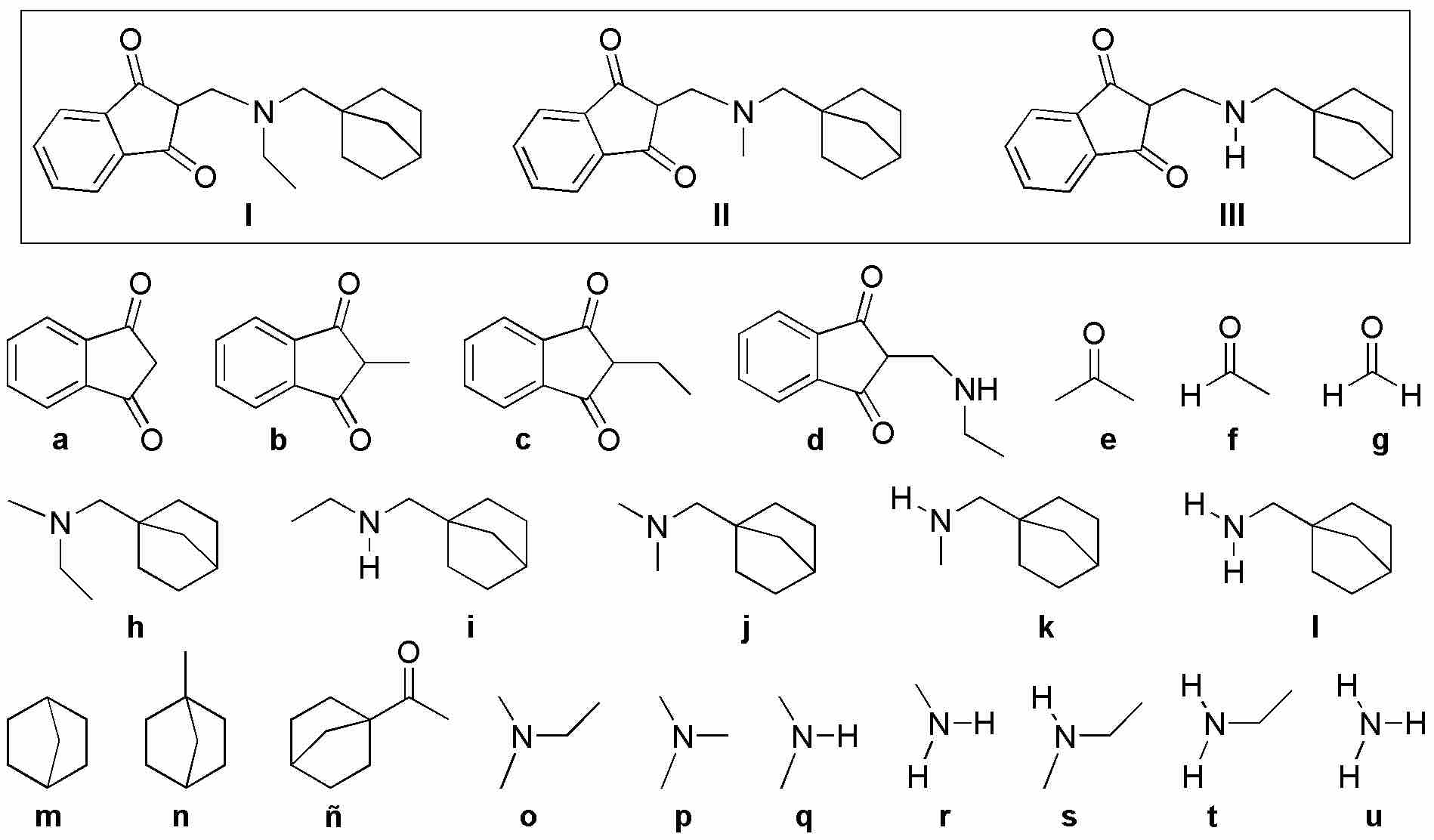
Indicate which compounds (a-u) you would use in a Mannich reaction to obtain the compounds (I-III) in the box.
Problem 18)
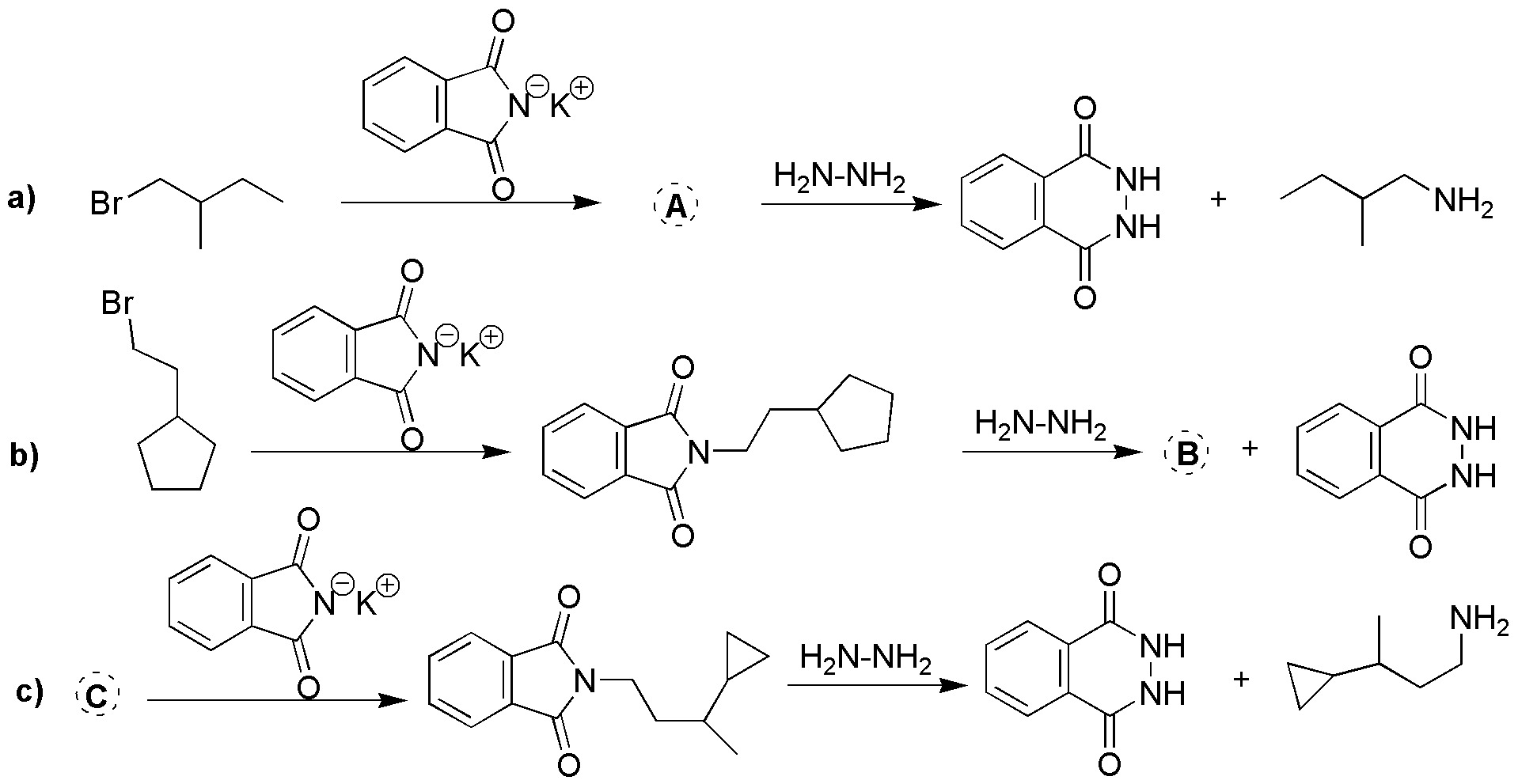
Complete the following Gabriel syntheses (a-c).
Problem 19)
What amines are obtained with the starting reagents (a-c), using Gabriel synthesis?

Problem 20)
Draw the structure of the compounds obtained when the following amines are treated with sodium nitrite in acidic medium.
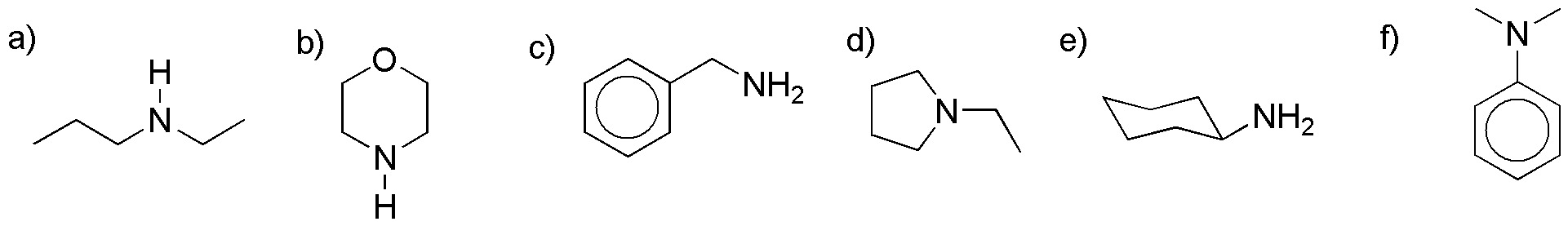
Problem 21)
How could the following transformations be carried out?
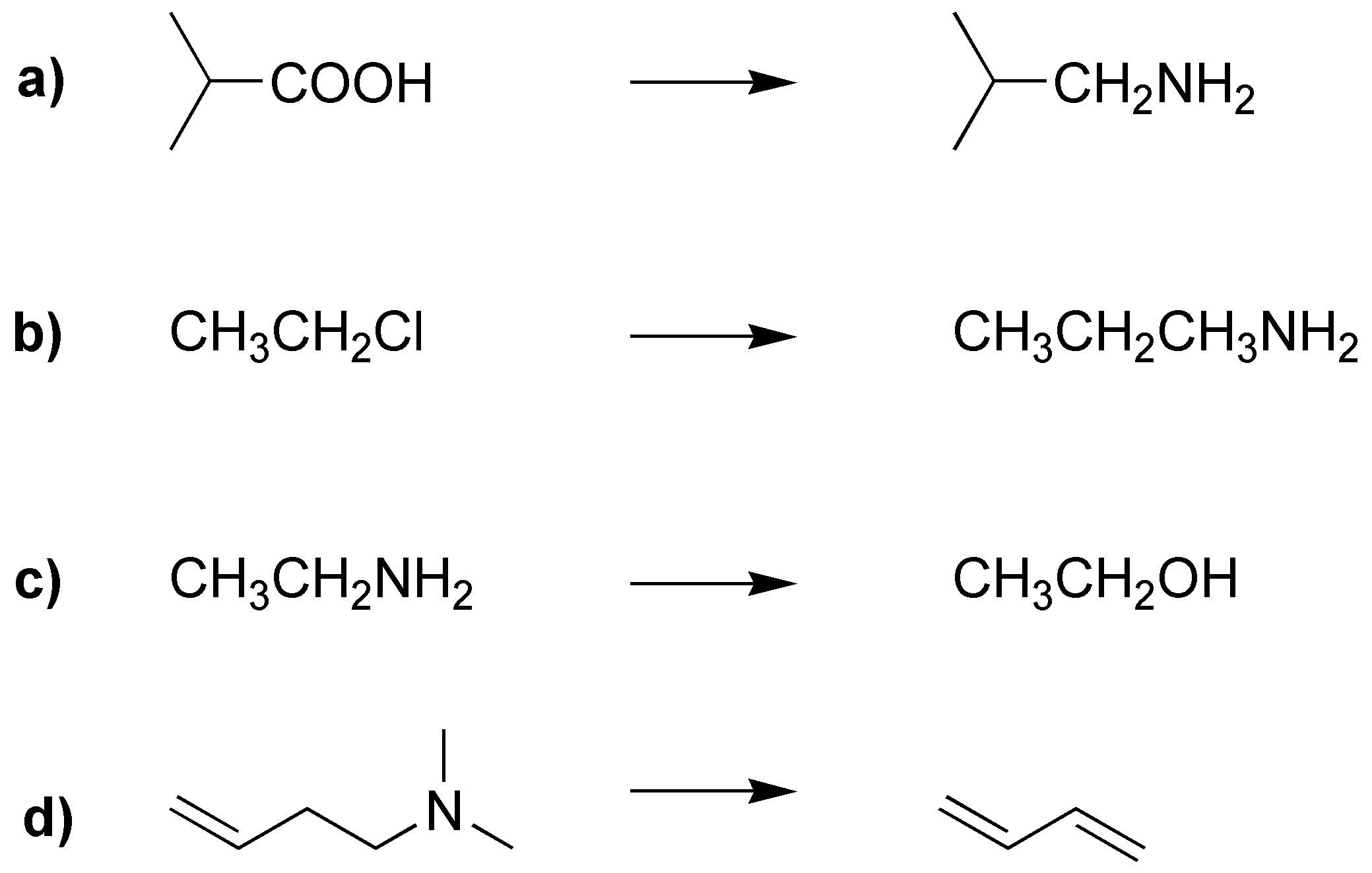
Problem 22)
What products would be obtained by Hofmann degradation of the following amines?
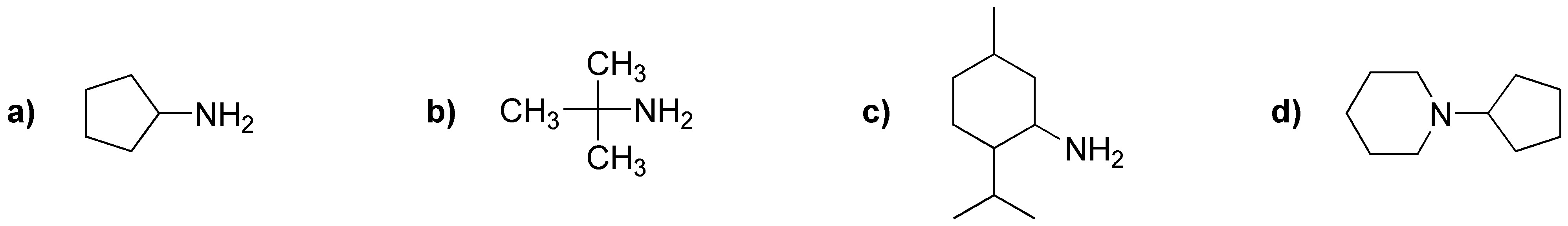
Problem 23)
Suggest a preparation of benzylamine using Gabriel synthesis.