Written by J.A Dobado | Last Updated on April 22, 2024
What is Kugelrohr distillation?
Kugelrohr distillation is a type of distillation that utilizes a unique piece of laboratory equipment known as a Kugelrohr.
It is a device that allows the distillation of small quantities of liquids (or low melting point solids) (<100 mg), including solvents with low boiling point as well as for drying samples. It is also used to separate polymeric substances or high melting point oils.
This distillation method is often used in the chemical and pharmaceutical industries to purify and isolate a wide range of compounds, including liquids, gases, and even solids.
The system has a series of glass spheres that are connected together. The balls are rotating and moving at the same time in front of a heat source, and the assembly is connected to a vacuum pump.
The samples can be observed directly and due to the transparency of the glass visualize how they pass from one ball to another (see Figure). When used to dry solid samples it is a substitute for a desiccator.
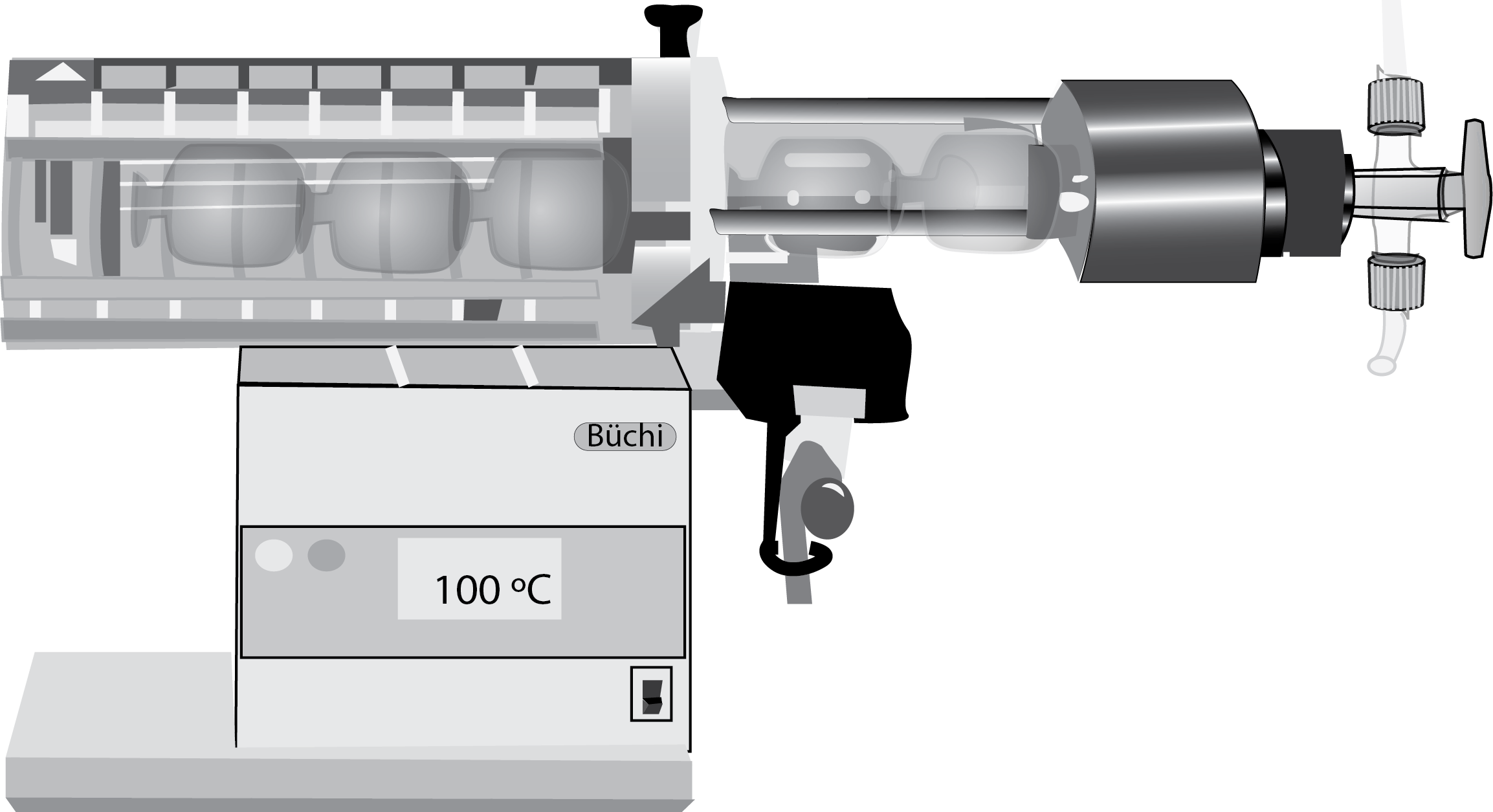
The Kugelrohr, also known as a “ball tube,” is a cylindrical glass tube with a spherical bulge in the middle. It is heated from the bottom, and the substance being distilled is placed in the spherical bulge. As the substance is heated, it vaporizes and travels through the tube, where it is cooled and condenses back into a liquid. This process allows the purest form of the substance to be collected at the end of the tube.
Step-by-step use
Follow these steps to perform a simple distillation:
- Use a Pasteur pipette to place the sample in the end bulb. If necessary, rinse the sample into the bulb and evaporate the solvent on a rotary evaporator.
- Connect two additional bulbs to the motor assembly using a straight length of glass tubing.
- Carefully insert the end bulb containing the sample into the oven and gently close the iris seal around the connecting joint.
- Rotate the bulbs to prevent bumping and speed up the distillation process.
- Gradually apply vacuum to prevent frothing and increase the oven temperature until distillation begins. Usually, a mist will appear in the first bulb to indicate the start of distillation. Adjust the temperature as needed to maintain a steady rate of distillation. If necessary, cool the collection bulb to efficiently condense the product.
- Once distillation is complete, allow the flasks to cool under an inert atmosphere. Then, remove the bulbs from the apparatus and recover the distillate. If washing the collection bulb with solvent is not required, clamp it in a vertical position and let its contents drain into a flask or sample vial.
To separate two compounds with a boiling point difference of 20-30 ºC, a method called fractionation can be used. This involves using three bulbs, where two of them are inserted into an oven. The most volatile component is distilled into the third bulb, and then the second bulb is withdrawn from the oven. The higher boiling fraction is then distilled into the second bulb.
Pros and cons of Kugelrohr distillation
One of the primary advantages of Kugelrohr distillation is its ability to distill substances at high temperatures without the risk of decomposition or other chemical reactions. This makes it ideal for the purification of heat-sensitive compounds, such as essential oils and fragrances. Additionally, the Kugelrohr is capable of producing highly pure and concentrated products, making it a popular choice for the production of high-quality chemical and pharmaceutical products.
One of the main limitations of Kugelrohr distillation is its relatively low throughput. It is not a particularly fast process, and it is not well-suited for the large-scale production of substances. However, it is an excellent choice for small-scale and laboratory-scale operations, as it allows for precise control and fine-tuning of the distillation process.
Summary
Kugelrohr distillation is a valuable tool for the purification and isolation of a wide range of substances. Its ability to distill at high temperatures and produce highly pure products make it a popular choice in the chemical and pharmaceutical industries, although its low throughput means it is more suitable for small-scale and laboratory-scale operations.
Video on Kugelrohr distillation
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2