Written by J.A Dobado | Last Updated on April 22, 2024
What is a rotary evaporator?
A common basic operation in the organic chemistry laboratory is the removal of a volatile organic solvent from a reaction mixture or a process such as liquid-liquid extraction. Although this operation could, in principle, be performed by simple distillation, the fastest and most convenient method is the use of a rotary evaporator (rotovap) for distillation under reduced pressure.
Basically, it consists of an electric motor, which causes the rotation of a tube attached to a ground glass-oint guide tube. A round-bottomed flask containing the solution is attached to the latter. This flask is partially immersed in a water bath, maintaining the rotation. The temperature of the bath should not exceed 35–40 °C for handling the most common organic solvents. Next to the system there is a condenser that circulates a liquid (water or antifreeze). This causes condensation of the solvent, which is recovered in a collector. The set is a closed system connected to a vacuum system (vacuum pump, water pump or vacuum circuit).
There are solutions that, when placed in a rotary evaporator, have a strong tendency to form foams or produce splashes that move up the guide tube to deposit in the indoor coil. This drawback is avoided by using a so-called foam breake. This is a glass adapter placed between the guide tube and the round-bottomed flask. It has two ground-glass joints: the female at the top, connected to the guide tube, and the male at the bottom, connected to the flask. The body consists of a glass sphere with elbow pipes or other device to prevent any liquid material in the flask from rising up the guide tube, and only the vapors do so.
General scheme
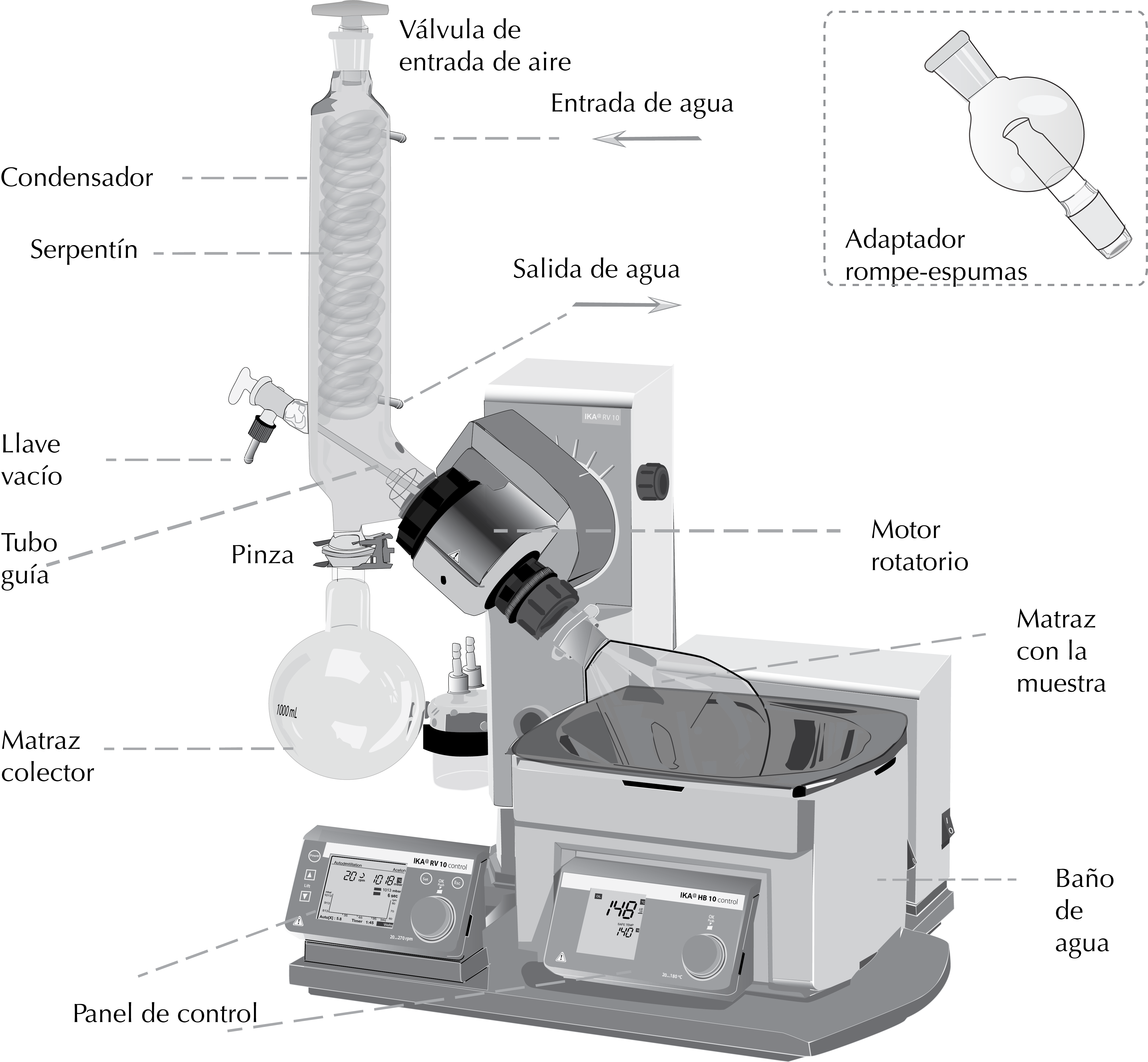
How to use a rotovap
- A round bottom flask is placed with the mixture that we want to evaporate the solvent, filling it at the most up to half of its capacity.
- Check that both the collector and the evaporator tube are clean, because if the collector contains a solvent of a lower b.p. than the one to be removed, the evaporation process is slowed down.
- Turn on the vacuum system, closing the air valve to the outside, and check by hand that there is vacuum in the guide tube.
- Lift the assembly using and attach the flask to the ground glass mouth of the guide tube, holding it with a clamp to prevent it from falling into the water bath.
- Open the coolant water intakes.
- Immerse the flask partially in the water bath and turn on the motor that rotates the flask. Adjust the speed of the rotation so that there is no projection of the liquid from the flask into the guide tube.
- Connect the vacuum source and close the tap that communicates the system with the outside.
- Using the jack, lower the assembly until the distillation flask is partially submerged in the water bath.
- Turn on the heating of the bath and heat to the minimum temperature necessary to achieve evaporation of the solvent.
- Continue the distillation until no more condensation of vapors is observed in the collector and the volume of the contents of the distillation flask no longer decreases.
- Lift the assembly until the flask is removed from the water bath.
- First disconnect the vacuum connection and then open the air valve to the outside.
- Stop the motor and remove the distillation flask from the mouth of the guide tube with rotating movements.
- Shut off vacuum and water condenser, and turn off the bathroom heating.
- Empty the contents of the collector and check that the guide tube is clean. If the tube is dirty, wash it with acetone.