Written by J.A Dobado | Last Updated on April 22, 2024
What is electronegativity?
The concept of electronegativity is originated from the experimental observation, for example, elements such as chlorine and fluoride exhibit a very strong tendency to give negative ions. On the other hand, other elements in group I, as there are electronegative, and is best described as electropositive (tendency to form positive ions).
This concept appears as one of the oldest chemical descriptors for predicting the reactivity of molecules. Berzelius (1779-1848), based on the concepts of electricity of his time, attempts to classify substances into electropositive and electronegative. He describes all chemical compounds as containing two electrically opposite components: the acidic, or electronegative, and the basic, or electropositive.
In 1932, Linus Pauling defines electronegativity as the ability of an atom to attract an electron to itself.
The scale of the coefficients of electronegativity is very useful because it measures the tendency of an atom to attract electrons towards itself.
Although there are different methods to estimate the electronegativity (for example, scales of Mulliken or Allred-Rochow), it is used commonly the scale developed by Pauling, which are tabulated relative values (without units).
On that scale, the most electronegative element (fluorine) is assigned the value greater positive 3,98, and the lower value of 0.7, is assigned to at least electronegative (francium). In some textbooks is assigned to the fluorine a value for the electronegativity of 4.0.
The values of the other atoms are in this range. In addition, the electronegativity of the hydrogen it is usually set to a value of 2.1 or 2.2 as shown in the following electronegativity chart.
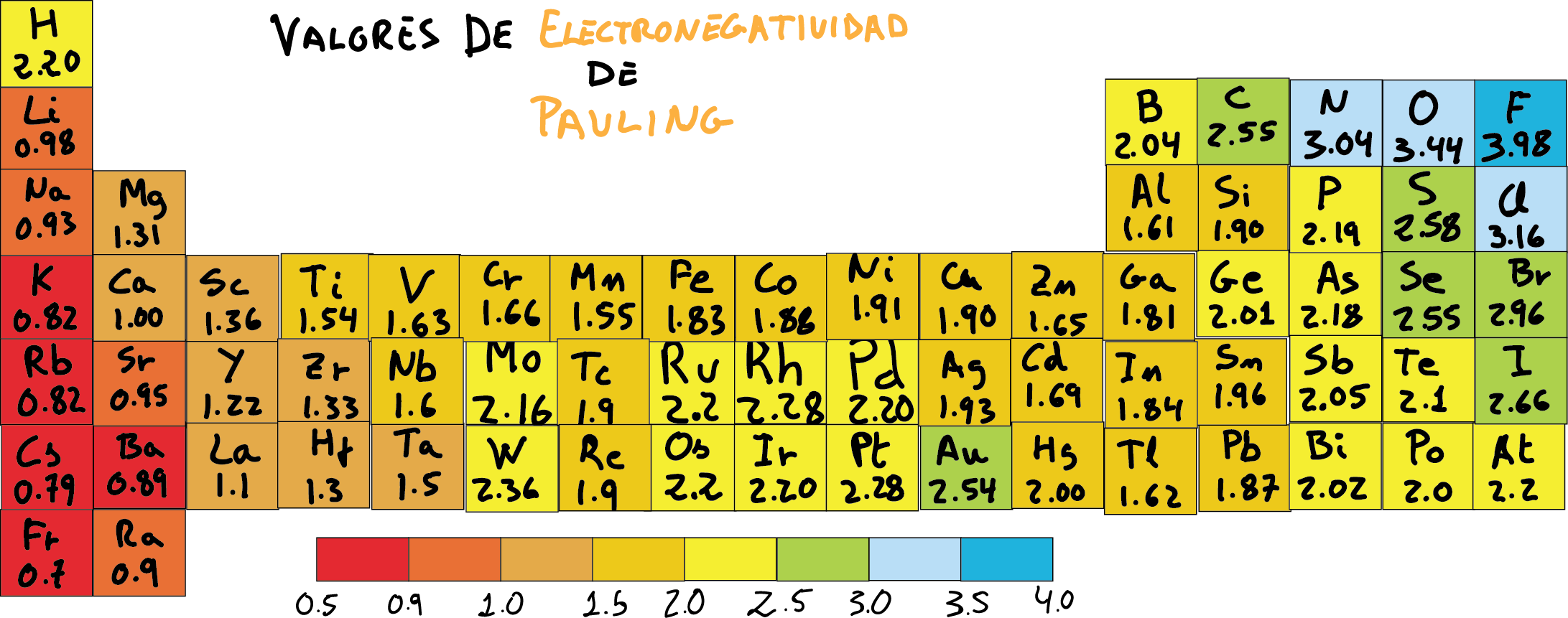
Pauling scale
| Name (symbol) | Electro- negativity (δIN) |
| Francium (Fr) | 0.7 |
| Cesium (Cs) | 0.79 |
| Potassium (K) | 0.82 |
| Rubidium (Rb) | 0.82 |
| Barium (Ba) | 0.89 |
| Radium (Ra) | 0.89 |
| Sodium (Na) | 0.93 |
| Strontium (Sr) | 0.95 |
| Lithium (Li) | 0.98 |
| Calcium (Ca) | 1 |
| Lanthanum (La) | 1.1 |
| Actinium (Ac) | 1.1 |
| Cerium (Ce) | 1.12 |
| Praseodymium (Pr) | 1.13 |
| Neodymium (Nd) | 1.14 |
| Samarium (Sm) | 1.17 |
| Gadolinium (Gd) | 1.2 |
| Yttrium (Y) | 1.22 |
| Dysprosium (Dy) | 1.22 |
| Holmium (Ho) | 1.23 |
| Erbium (Er) | 1.24 |
| Thulium (Tm) | 1.25 |
| Lutetium (Lu) | 1.27 |
| Plutonium (Pu) | 1.28 |
| Hafnium (Hf) | 1.3 |
| Thorium (Th) | 1.3 |
| Americium (Am) | 1.3 |
| Curium (Cm) | 1.3 |
| Berkelio (Bk) | 1.3 |
| Californium (Cf) | 1.3 |
| Einsteinium (Es) | 1.3 |
| Fermium (Fm) | 1.3 |
| Mendelevium (Md) | 1.3 |
| Nobelium (No) | 1.3 |
| Laurencium (Lr) | 1.3 |
| Magnesium (Mg) | 1.31 |
| Zirconium (Zr) | 1.33 |
| Scandium (Sc) | 1.36 |
| Neptunium (Np) | 1.36 |
| Uranium (U) | 1.38 |
| Tantalum (Ta) | 1.5 |
| Protactinium (Pa) | 1.5 |
| Titanium (Ti) | 1.54 |
| Manganese (Mn) | 1.55 |
| Beryllium (Be) | 1.57 |
| Niobium (Nb) | 1.6 |
| Aluminum (Al) | 1.61 |
| Thallium (Tl) | 1.62 |
| Vanadium (V) | 1.63 |
| Zinc (Zn) | 1.65 |
| Chromium (Cr) | 1.66 |
| Cadmium (Cd) | 1.69 |
| Indium (In) | 1.78 |
| Gallium (Ga) | 1.81 |
| Iron (Fe) | 1.83 |
| Cobalt (Co) | 1.88 |
| Silicon (Si) | 1.9 |
| Copper (Cu) | 1.9 |
| Technetium (Tc) | 1.9 |
| Rhenium (Re) | 1.9 |
| Nickel (Ni) | 1.91 |
| Silver (Ag) | 1.93 |
| Tin (Sn) | 1.96 |
| Mercury (Hg) | 2 |
| Polonium (Po) | 2 |
| Germanium (Ge) | 2.01 |
| Bismuth (Bi) | 2.02 |
| Boron (B) | 2.04 |
| Antimony (Sb) | 2.05 |
| Tellurium (Te) | 2.1 |
| Molybdenum (Mo) | 2.16 |
| Arsenic (As) | 2.18 |
| Phosphorus (P) | 2.19 |
| Hydrogen (H) | 2.2 |
| Ruthenium (Ru) | 2.2 |
| Palladium (Pd) | 2.2 |
| Osmium (Os) | 2.2 |
| Iridium (Ir) | 2.2 |
| Astatine (At) | 2.2 |
| Rhodium (Rh) | 2.28 |
| Platinum (Pt) | 2.28 |
| Lead (Pb) | 2.33 |
| Tungsten (W) | 2.36 |
| Gold (Au) | 2.54 |
| Carbon (C) | 2.55 |
| Selenium (Se) | 2.55 |
| Sulfur (S) | 2.58 |
| Xenon (Xe) | 2.6 |
| Iodine (I) | 2.66 |
| Bromine (Br) | 2.96 |
| Nitrogen (N) | 3.04 |
| Chlorine (Cl) | 3.16 |
| Oxygen (O) | 3.44 |
| Fluor (F) | 3.98 |
Allred-Rochow scale of electronegativity
Unlike the scales of electronegativity Pauling and Mulliken, based on the coefficients of Allred-Rochow it is much more accurate and is widely accepted.
In this the coefficient of electronegativity is defined as: the force of electrostatic attraction that you experience an atom (I effective mass nuclear Zef) for an electron at a distance from the nucleus equal to the covalent radius of this atom (valence electron).
Allred-Rochow’s electronegativity coefficients, χAR, are obtained from the following formula:
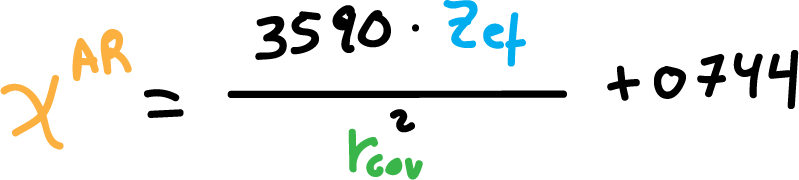
Where, Zef is the effective mass nuclear, and rcov is the covalent radius, the corresponding atom.
In general, the coefficients of electronegativity Allred-Rochow in the periodic table vary in a manner that will increase as we move to the right and decrease as we go down the rows. This is the opposite of the increase in the covalent radius, and in the same way that the trends in the first ionization energy.
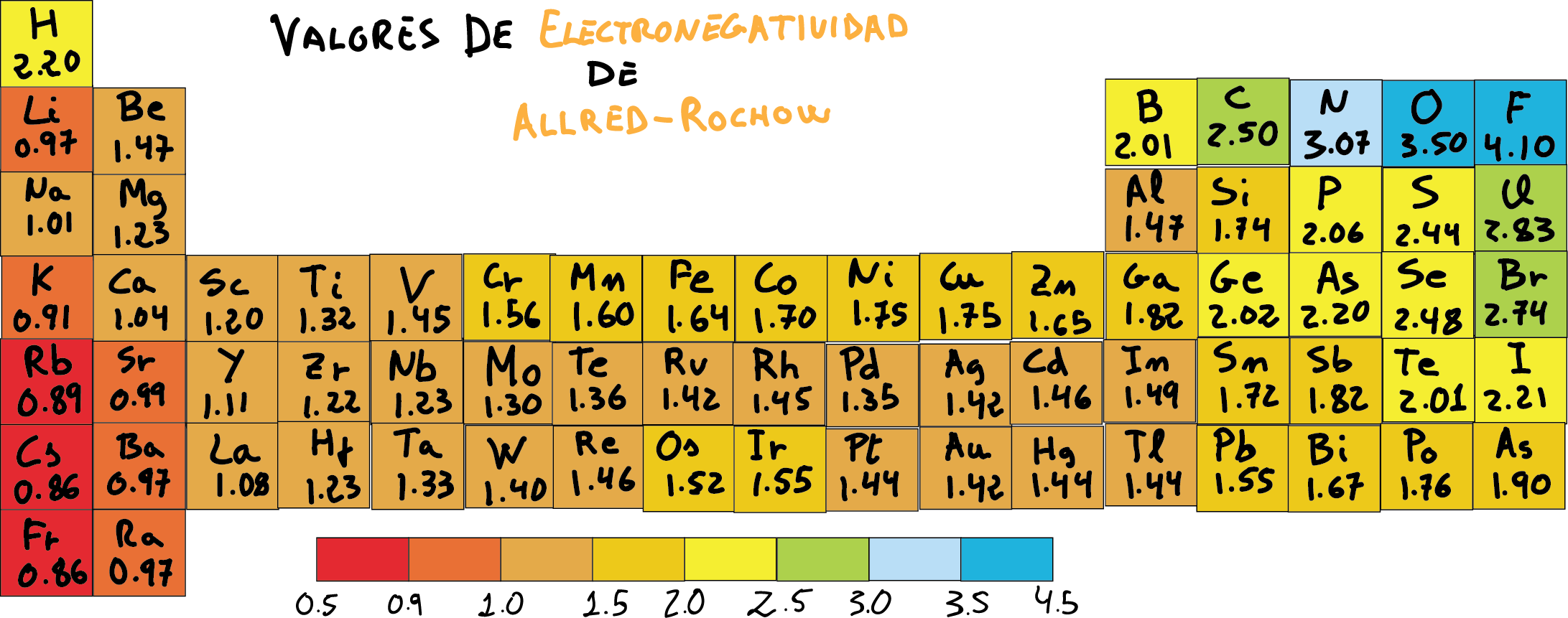
Allred-Rochow electronegativity chart
| Name (symbol) | Electro- negativity (χAR) |
| Francium (Fr) | 0.86 |
| Cesium (Cs) | 0.86 |
| Rubidium (Rb) | 0.89 |
| Potassium (K) | 0.91 |
| Barium (Ba) | 0.97 |
| Radium (Ra) | 0.97 |
| Lithium (Li) | 0.97 |
| Strontium (Sr) | 0.99 |
| Sodium (Na) | 1.01 |
| Boron (B) | 1.01 |
| Calcium (Ca) | 1.04 |
| Lanthanum (La) | 1.08 |
| Yttrium (Y) | 1.11 |
| Scandium (Sc) | 1.20 |
| Zirconium (Zr) | 1.22 |
| Hafnium (Hf) | 1.23 |
| Magnesium (Mg) | 1.23 |
| Niobium (Nb) | 1.23 |
| Molybdenum (Mo) | 1.30 |
| Titanium (Ti) | 1.32 |
| Tantalum (Ta) | 1.33 |
| Palladium (Pd) | 1.35 |
| Tellurium (Te) | 1.36 |
| Tungsten (W) | 1.40 |
| Silver (Ag) | 1.42 |
| Ruthenium (Ru) | 1.42 |
| Gold (Au) | 1.42 |
| Thallium (Tl) | 1.44 |
| Mercury (Hg) | 1.44 |
| Platinum (Pt) | 1.44 |
| Vanadium (V) | 1.45 |
| Rhodium (Rh) | 1.45 |
| Cadmium (Cd) | 1.46 |
| Rhenium (Re) | 1.46 |
| Beryllium (Be) | 1.47 |
| Aluminum (Al) | 1.47 |
| Indium (In) | 1.49 |
| Osmium (Os) | 1.52 |
| Iridium (Ir) | 1.55 |
| Lead (Pb) | 1.55 |
| Chromium (Cr) | 1.56 |
| Manganese (Mn) | 1.60 |
| Iron (Fe) | 1.64 |
| Zinc (Zn) | 1.65 |
| Bismuth (Bi) | 1.67 |
| Cobalt (Co) | 1.70 |
| Tin (Sn) | 1.72 |
| Silicon (Si) | 1.74 |
| Copper (Cu) | 1.75 |
| Nickel (Ni) | 1.75 |
| Polonium (Po) | 1.76 |
| Gallium (Ga) | 1.82 |
| Antimony (Sb) | 1.82 |
| Technetium (Tc) | 1.9 |
| Astatine (At) | 1.90 |
| Germanium (Ge) | 2.02 |
| Phosphorus (P) | 2.06 |
| Arsenic (As) | 2.20 |
| Hydrogen (H) | 2.20 |
| Iodine (I) | 2.21 |
| Sulfur (S) | 2.44 |
| Selenium (Se) | 2.48 |
| Carbon (C) | 2.50 |
| Xenon (Xe) | 2.6 |
| Bromine (Br) | 2.74 |
| Chlorine (Cl) | 2.83 |
| Nitrogen (N) | 3.07 |
| Oxygen (O) | 3.50 |
| Fluor (F) | 4.10 |
Video on the electronegativity chart
Frequently Asked Questions on the Electronegativity chart
- What is electronegativity?
The concept of electronegativity is originated from the experimental observation, for example, elements such as chlorine and fluoride exhibit a very strong tendency to give negative ions. On the other hand, other elements in group I, as there are electronegative, and is best described as electropositive (tendency to form positive ions).
- What is the Allred-Rochow’s electronegativity scale?
Unlike the Pauling and Mulliken electronegativity scales, the one based on the Allred-Rochow coefficients is much more accurate and is widely accepted.