Go to the page with the solutions to the problems.
Aromatic Substitution Reactions in Benzene and Derivatives – list of problems
Problem 1)
Indicate which of the following structures will be aromatic and which will not:
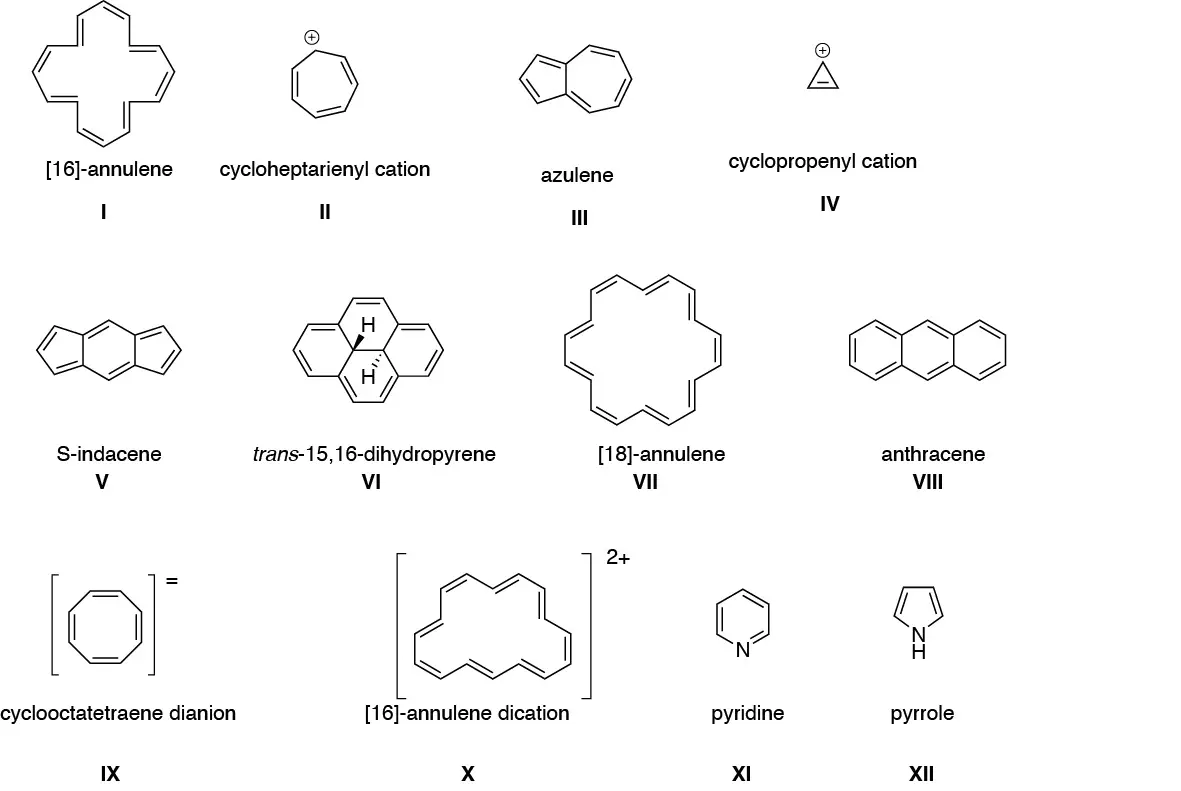
Problem 2)
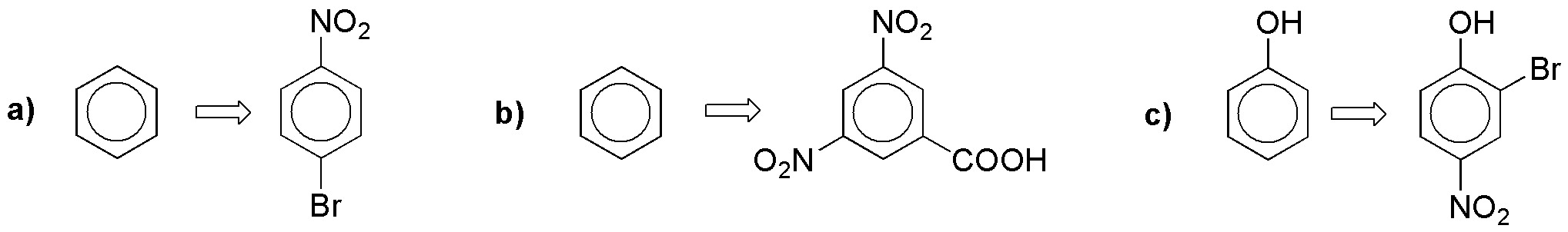
Prepare from benzene the following alkylenes in as few steps as possible.

Problem 3)
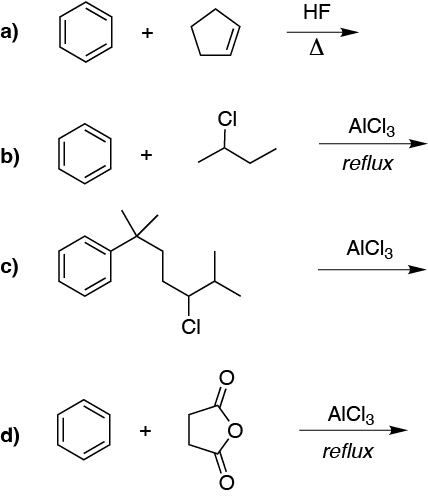
Prepare the following bicyclic compounds from benzene:
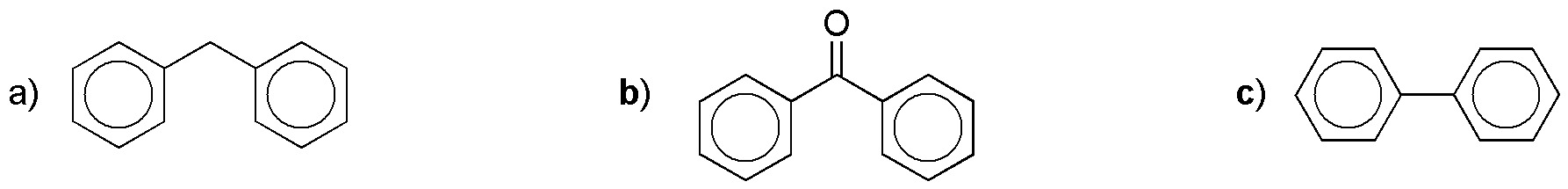
Problem 4)
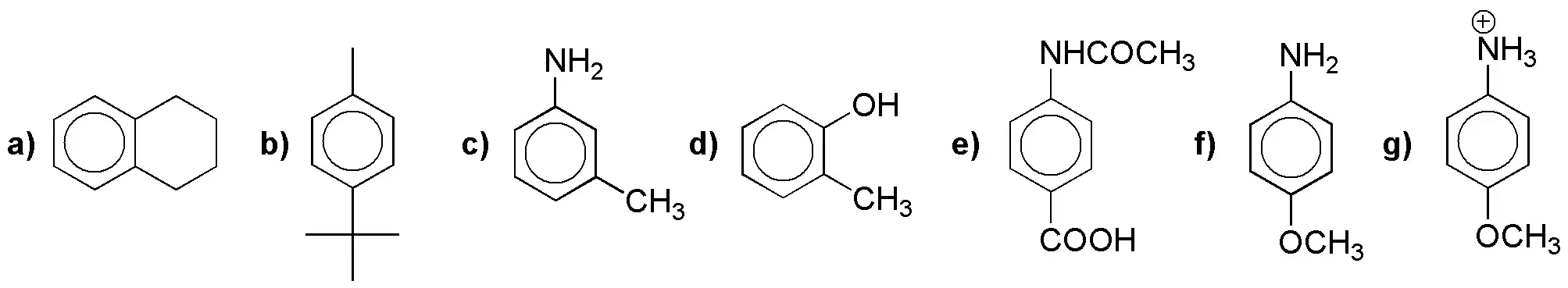
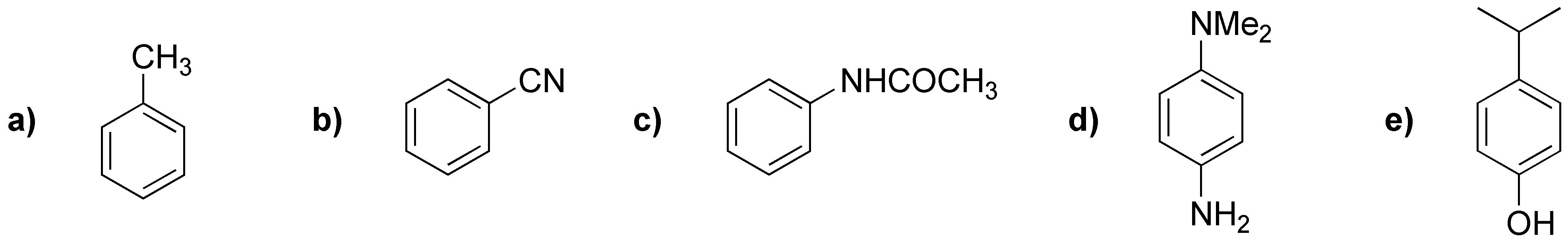
Rank the following monosubstituted arenes in order of increasing rate of electrophilic aromatic substitution reaction.
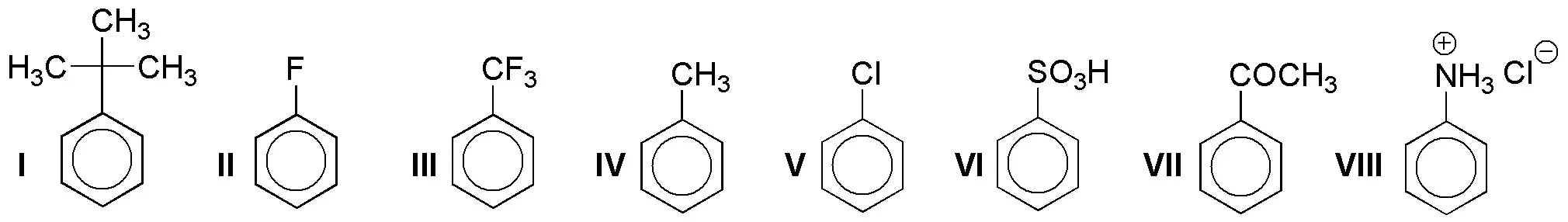
Problem 5)
Describe a procedure for the nitration of aniline.
Problem 6)
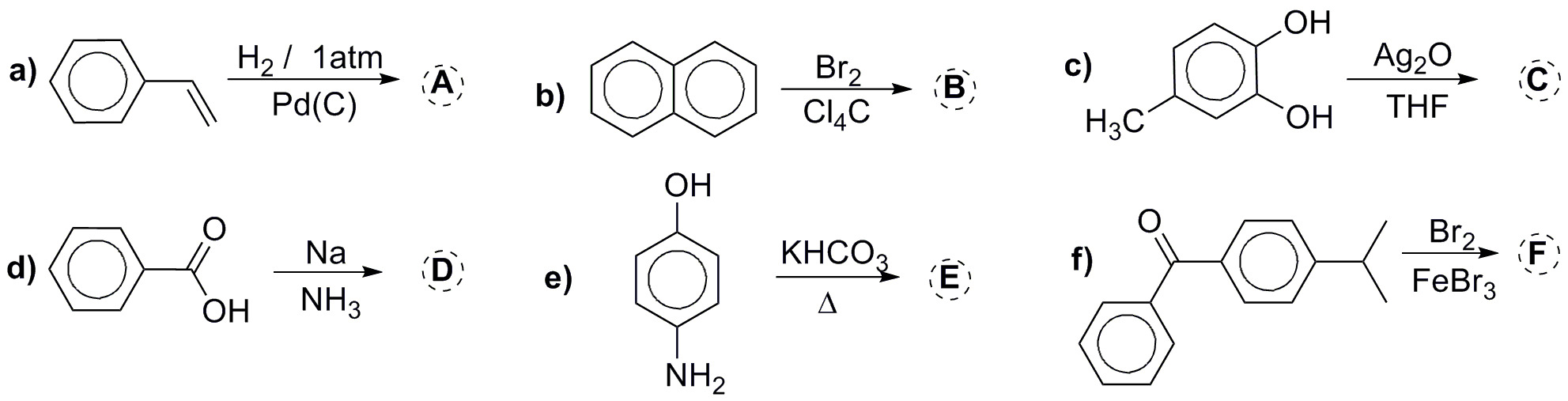
Explain in a reasoned manner the following transformations, single or multistep.
Problem 7)
Justify in a reasoned way the orientation of the electrophilic aromatic substitution reaction in the following cases, taking as reference the rate of benzene:
Problem 8)
Predict the major product in the following reactions:
Problem 9)
Rank in order of increasing rate of nucleophilic aromatic substitution reaction rate the following compounds:
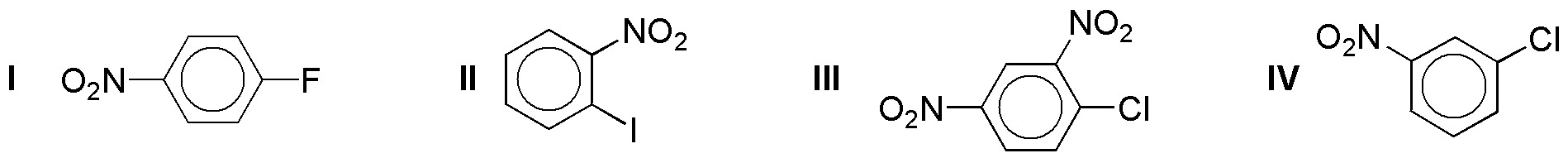
Problem 10)
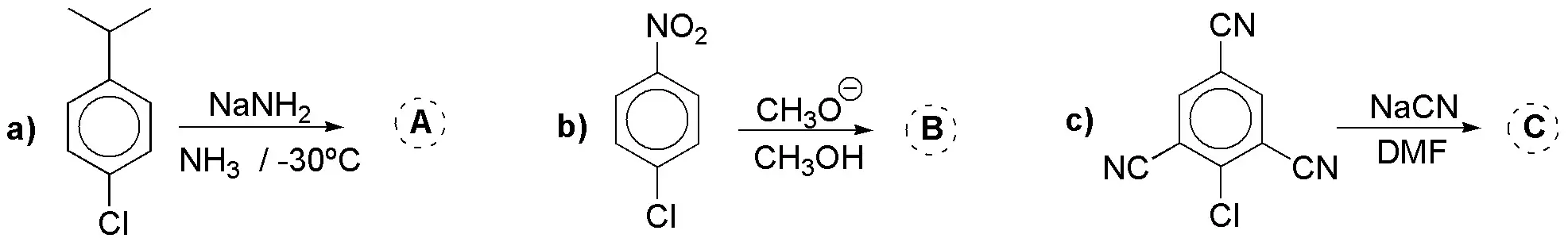
Justify the result of the following reactions:
Problem 11)
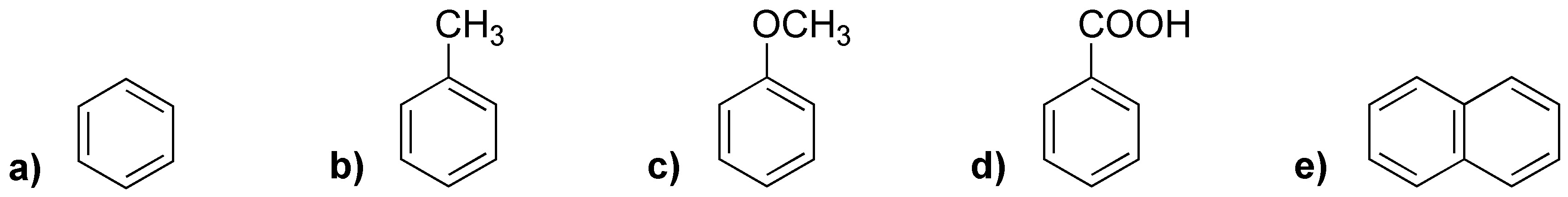
Rank the following compounds in order of increasing rate of electrophilic aromatic substitution reaction.
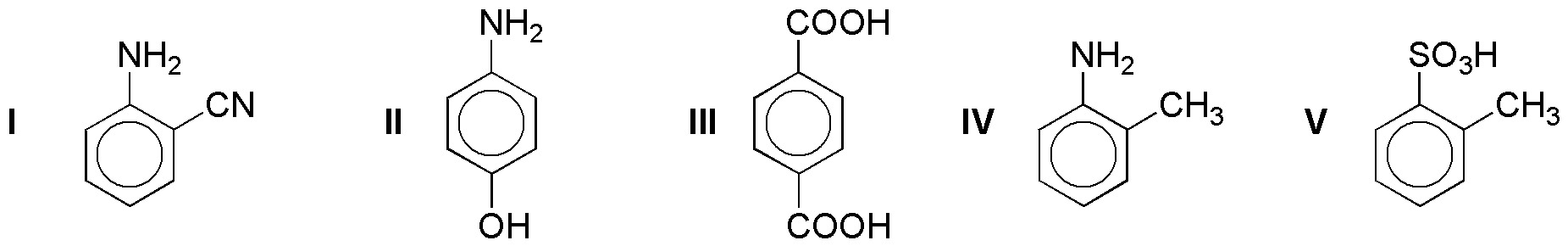
Problem 12)
Indicate which groups in the following series are meta-directing:
a) -CH3; b) -CH2Cl; c) -COOH; d) -SO3H; e) -CONH2; f) -NO; g) -NHCH3; h) -NHCOCH3; i) -OCH3; j) -ONa
Problem 13)
Explain the difference in reactivity between aniline and acetanilide in the bromination reaction.
Problem 14)
Predict the orientation of the Friedel-Crafts acylation reaction on the following substrates:
(a) 1,3-dinitrobenzene; (b) 1,3-dibromobenzene; (c) p-nitroaniline; (d) p-nitrophenol; (e) methyl 3-nitrobenzoate; (f) p-cresol; (g) 1-bromo-3-nitrobenzene.
Problem 15)
State with reasoning why the NO2 group is meta-directing while the NO group is ortho– and para-directing.
Problem 16)
Indicate for the following pairs of compounds, which one will present higher reaction rate (electrophilic aromatic substitution) against the same nucleophile?
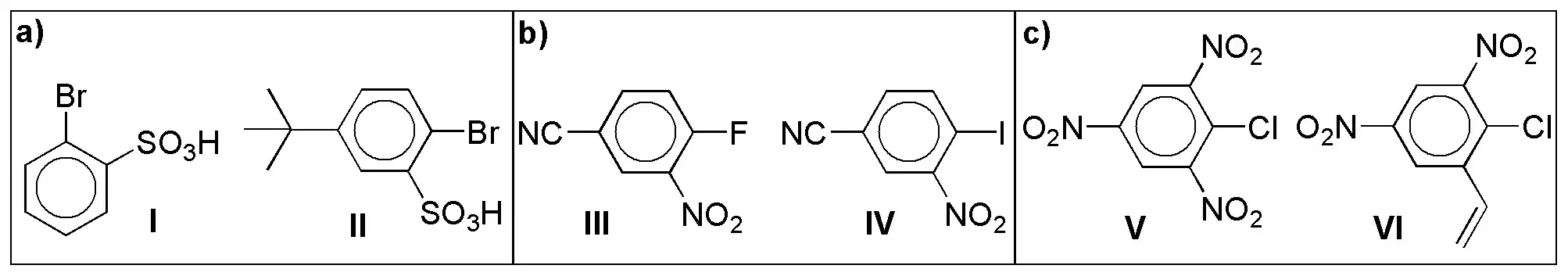
Problem 17)
Indicate which of the following products are aromatic or antiaromatic:
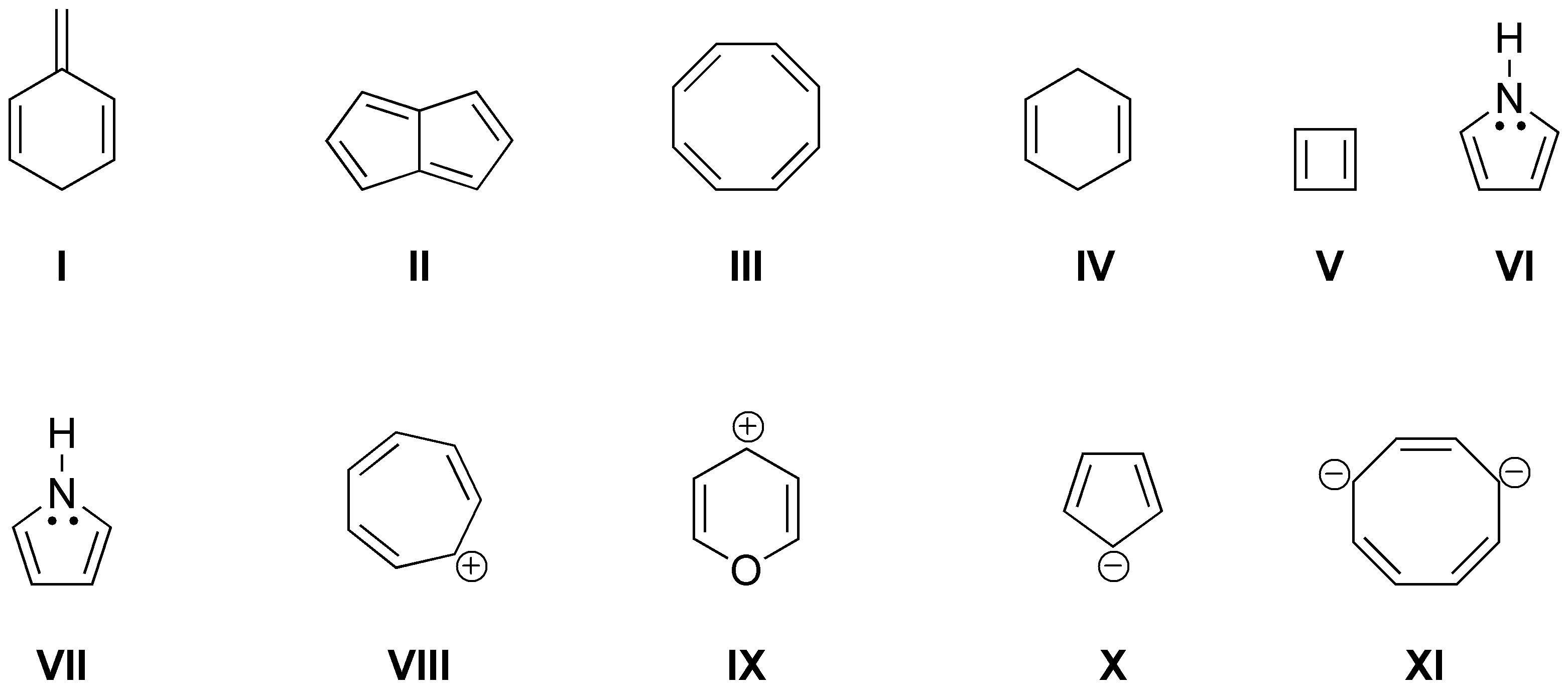
Problem 18)
Predict what will be the monosubstitution product of the following reactions:
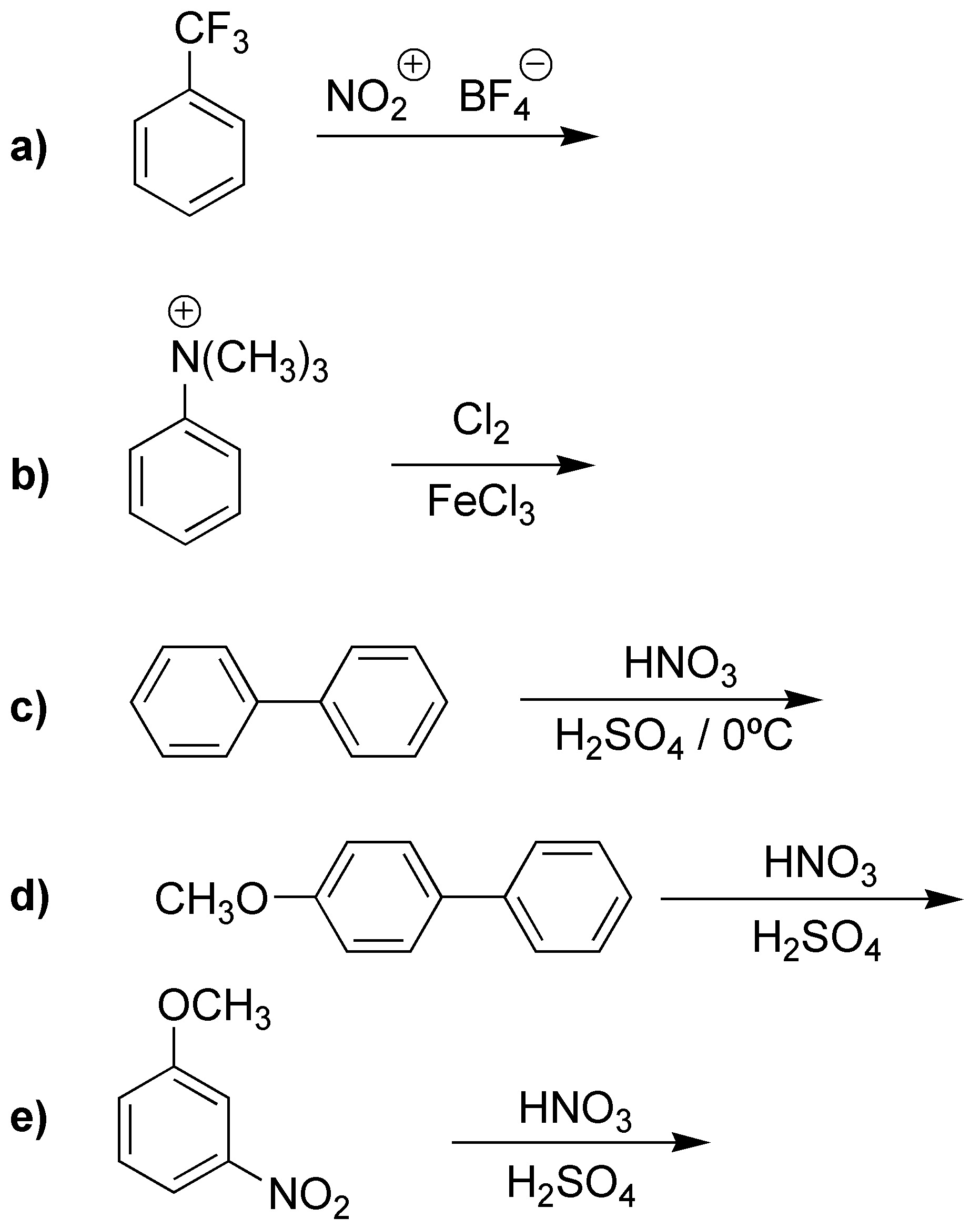
Problem 19)
Indicate at which position the monobromination of the following products can be expected to occur:
Problem 20)
Complete the following reactions:
Problem 21)
Compounds A, B and C are the three possible xylenes (dimethylbenzenes). Indicate what would be the structure of each of them taking into account that the nitration of them produces: one mononitrated derivative in the case of A, two mononitrated derivatives in the case of B and three in the case of C.
Problem 22)
Why is the nitration of toluene easier than that of benzene?
Problem 23)
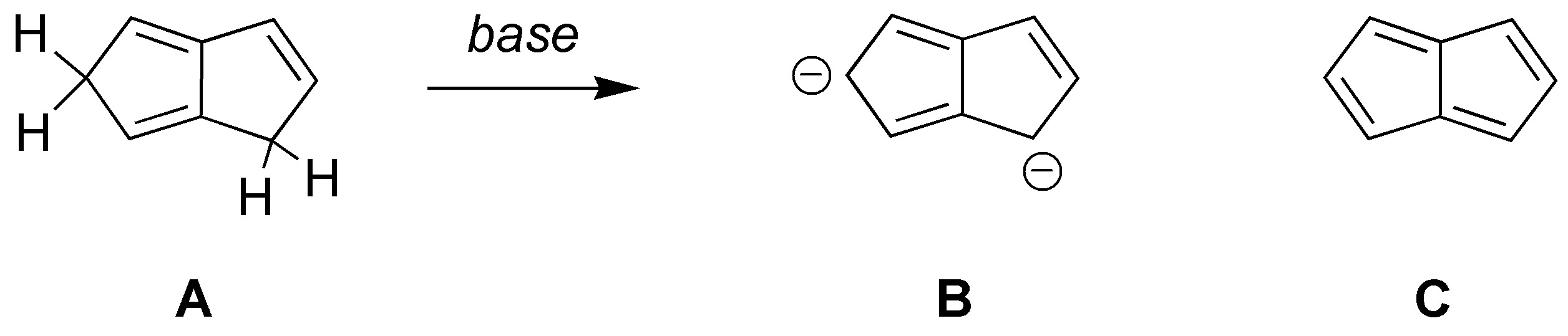
Explain why, when triene A is treated with a base the dianion B is produced which is stable, while the tetrenic product C (pentalene) is an unstable product.
Problem 24)
What would be the Birch reduction product of the following compounds:
Problem 25)
Predict the product formed in the following transformations:
Problem 26)
How could 4-nitrotoluene be prepared from benzene?