Written by J.A Dobado | Last Updated on April 22, 2024
How is the melting point of a solid measured?
The melting point (m.p.) of a substance is the temperature at which that solid changes to a liquid state (melts). This physical property is easy to measure, is characteristic of a specific substance and serves as an index of purity.
The m.p. is hardly affected by pressure changes. Melting of a substance generally occurs in the range of a few degrees (ºC) and, with increasing amounts of impurities in a solid, the m.p. decreases and the range in which the solid changes to liquid increases.
The m.p. of common pure organic compounds have values below 300 ºC and can be found in different databases and manuals. In order to correctly determine the m.p. of an organic compound, it is necessary to start from a dry sample that is free of solvent residues.
Melting point measurement devices
There are different types of devices to measure the melting point, and they are listed below:
Thiele tube
Historically, the simplest of the devices for measuring the melting point is the Thiele tube.
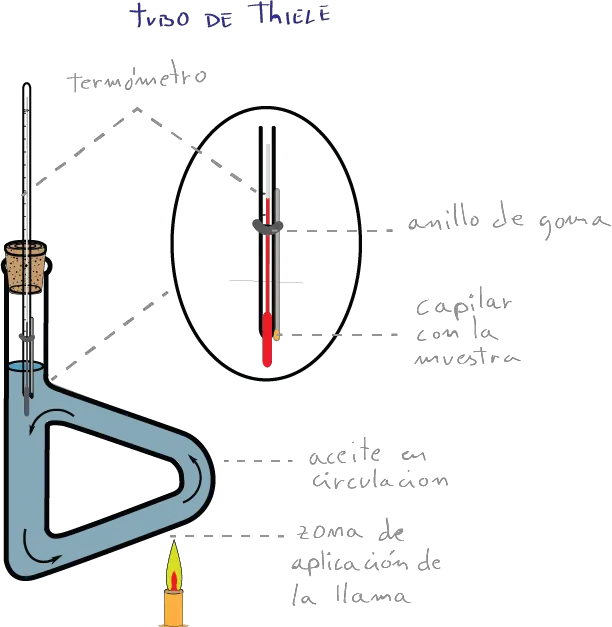
It is a heat-resistant glass tube containing a fluid (typically a mineral oil) with a stopper along with a thermometer with an opening through which a capillary containing the sample is placed.
Capillary is introduced with the sample. The Thiele tube is heated with a gas burner until melting of the solid in the capillary is seen.
The thermometer and capillary must not be in contact with the glass tube.
Electronic melting point apparatus
The Thiele tube has been replaced in most laboratories by electronic melting point measuring devices. These devices are safer and more accurate.

There are different designs and models on the market, most of them digital, but they all work in a similar way.
The simplest model has a hole through which the capillary is inserted in front of a sight glass.
The rate at which the temperature rises is adjustable and the melting point is determined by direct eye inspection (viewing the melting of the solid in the capillary through the sight glass).
Instead of having a fluid to transmit heat, some devices have a metal (aluminum) block.
Other more specific models use a microscope with a metal block, a resistor, a potentiometer and a thermometer.
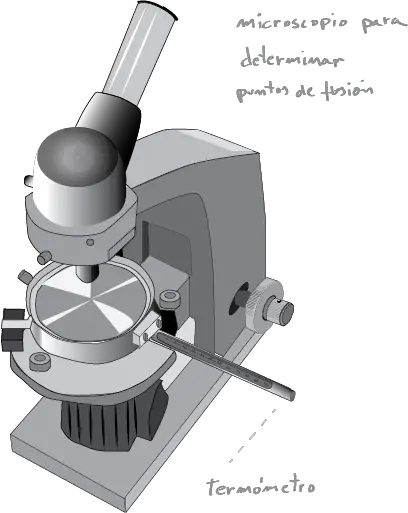
The sample is placed between two glass plates and gradually heated. This procedure is more accurate and allows the observer to see if there are other significant changes in the sample, such as softening or changes in color or homogeneity.
Sample preparation
The first step in determining the melting point (m.p.) of a solid is to place a few crystals of it in a glass capillary closed at one end (m.p. capillary tubes are commercially available).
To do this, a small amount of solid is placed on a watch glass or in a Petri dish, and the capillary tube is filled with the solid 2 or 3 mm high. Then, the capillary with the solid is inverted and dropped into a piece of hollow glass rod that holds it on the benchtop. Thus, the solid is compacted in the closed end of the capillary. Once the sample has been prepared in this way, the capillary is placed in the corresponding melting point apparatus.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2