Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the solutions to the problems.
Alcohols, Ethers and Oxiranes – problem list
Problem 1)
Classify the following alcohols into primary, secondary and tertiary alcohols.
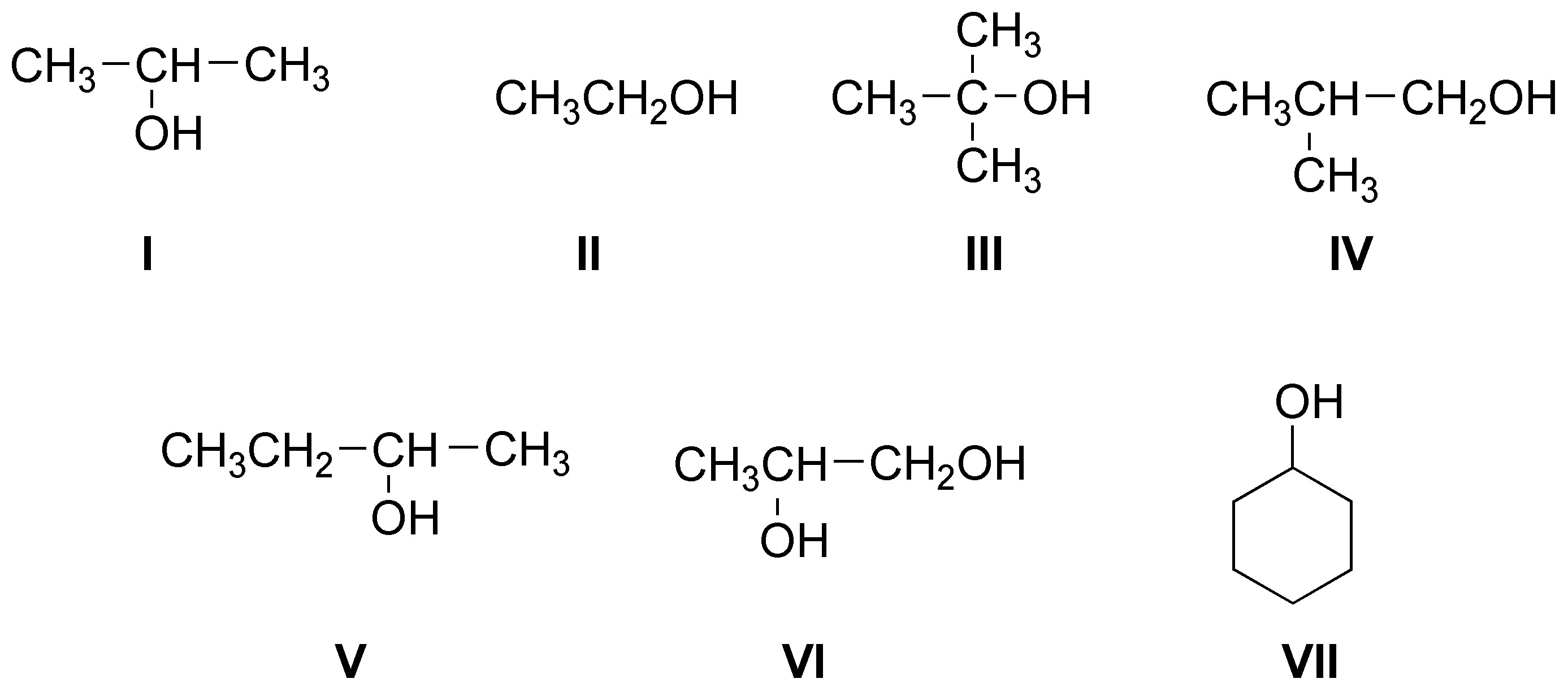
6.2) Indicate which reagents (a-f) give with propanol and tert-butanol the corresponding alkoxide.
a) NH3; b) Nao; c) NaOH; d) NaSO4; e) NaNH2; f) NaH
Problem 3)
When 2-methyl-2-butanol is treated with hydrobromic acid a single compound is obtained. Write the scheme of the reaction and propose a mechanism.
Problem 4)
If 1-pentanol is treated with hydrobromic acid a halogenated derivative is obtained. What reaction mechanism predominates in this transformation? Compare the results obtained with the previous problem.
Problem 5)
When HCl is used instead of HBr on 2-pentanol, it is necessary to add ZnCl2 to obtain good yields.Justify the result and propose a mechanism compatible with these facts.
Problem 6)
When 1-butanol is treated with H2SO4, 2-butene is obtained as the major product. Justify the result in a reasoned way.
Problem 7)
Draw the structures of the major product obtained in each case, by treatment of the following alcohols with a mineral acid such as H3PO4.
a) butan-1-ol; b) 2-methylcyclohexanol; c) 2-methylpentan-3-ol; d) (1S,3S)-1-isopropyl-3-methylcyclohexanol.
Problem 8)
What alcohols can give the following alkenes by dehydration reactions?
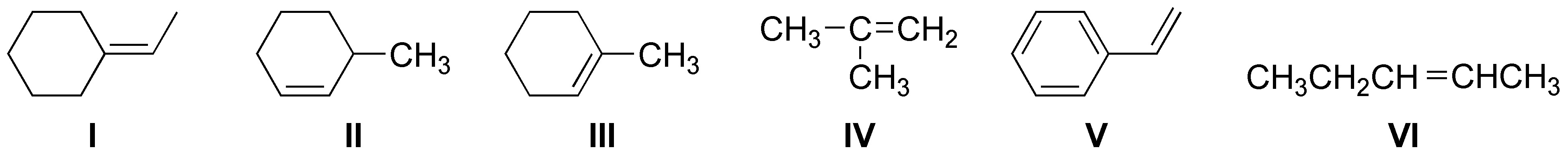
Problem 9)
Complete the following reactions and associate, to the corresponding ones, the concepts: Racemization, Configuration inversion, Reduction, Radical process, Esterification, Oxidation and Configuration retention.
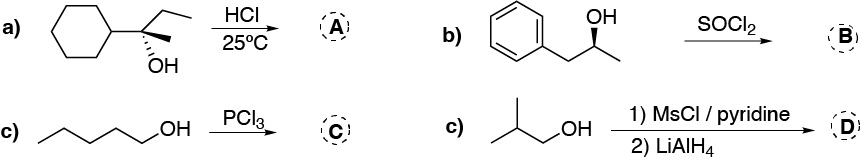
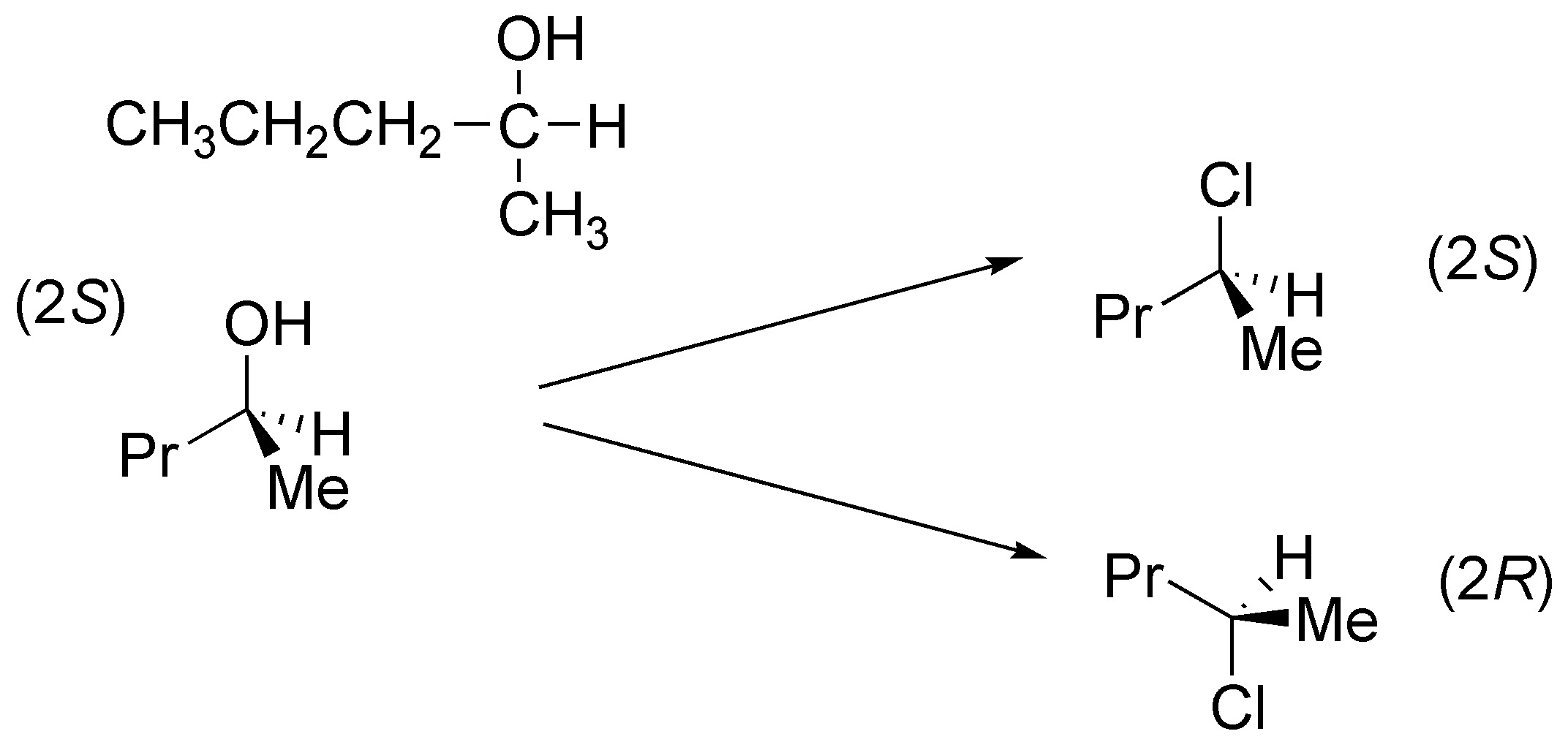
Problem 10)
Design a procedure for obtaining from (S)-pentan-2-ol the R and S stereoisomers of 2-chloropentane independently.
Problem 11)
Complete the following reactions, indicating the reagent(s) that allow the following transformations to be carried out:
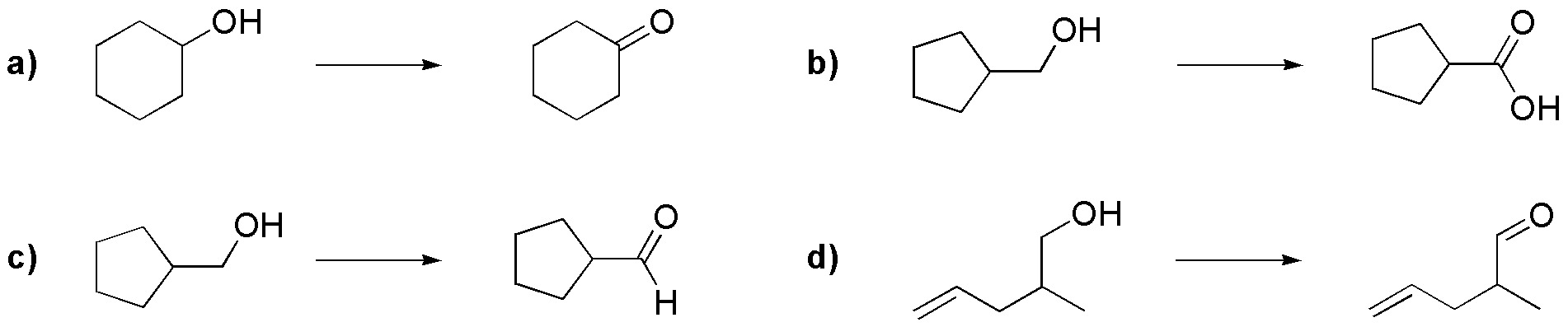
Problem 12)
Describe the structures of the compounds formed when 2-methylprop-2-en-1-ol is treated independently with the following reagents, as well as the mechanisms of the process f).
- a) PCC/ CH2Cl2
- b) LiAlH4/ THF and aqueous NH4Cl
- c) Thionyl chloride in pyridine
- d) Jones’ Reagent
- e) benzoyl chloride / pyridine
- f) Carbon tetrabromide and triphenylphosphine
- g) Oxalyl chloride / DMSO
- h) Sodium hydride / benzyl bromide / THF
Problem 13)
When propan-1-ol is treated with sodium followed by 1-bromopropane, a product A is obtained. Represent its structure and describe the mechanism of the reactions that occur.
Problem 14)
Choose the most suitable starting alcohol to prepare the following ethers by Williamson synthesis.
![]()
Problem 15)
The opening in aqueous acid medium of an oxirane A leads to the formation of 1,2-ethanediol. Draw its structure.
Problem 16)
An oxirane is treated with ethanol in acid medium and produces 3-ethoxy-2-butanol. Deduce its structure.
Problem 17)
Complete the following reactions:
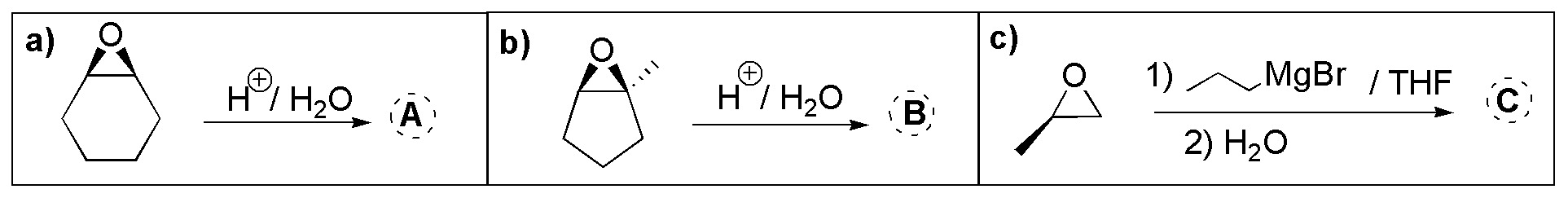
Problem 18)
Choose the epoxides you consider suitable as starting products, as well as the nucleophiles needed to prepare the following compounds.
Problem 19)
Rank the following epoxides in increasing order of reaction rate, when treated with aqueous sulfuric acid.
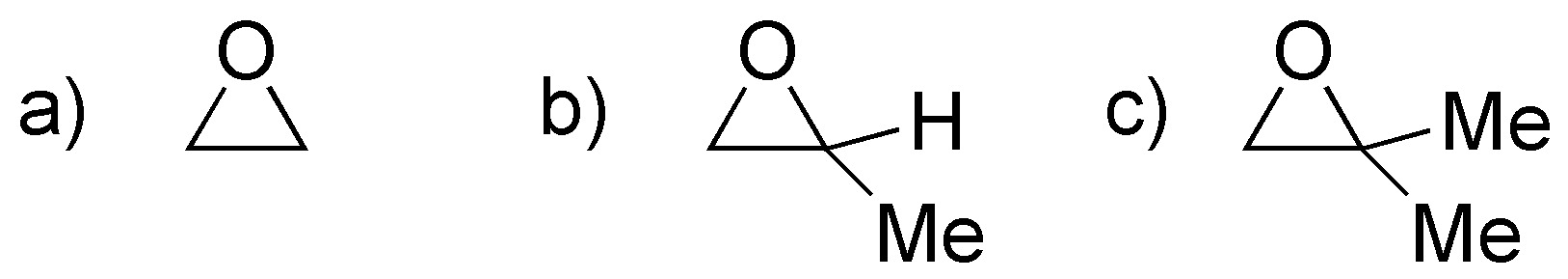
Problem 20)
Predict the major product in each of the following transformations.
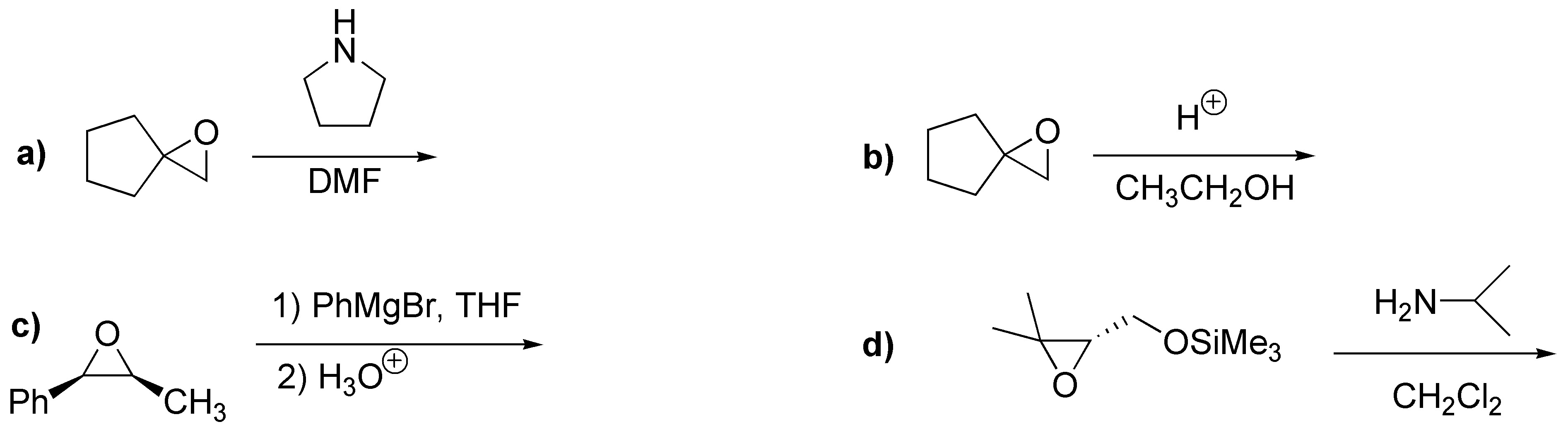
Problem 21)
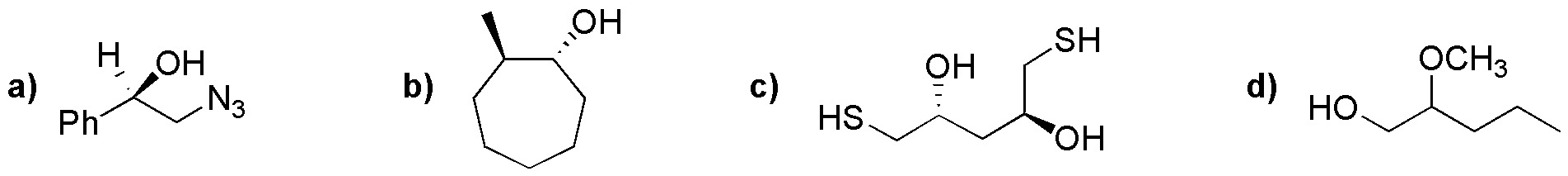
Identify and name the derivatives of alcohols in the following structures:
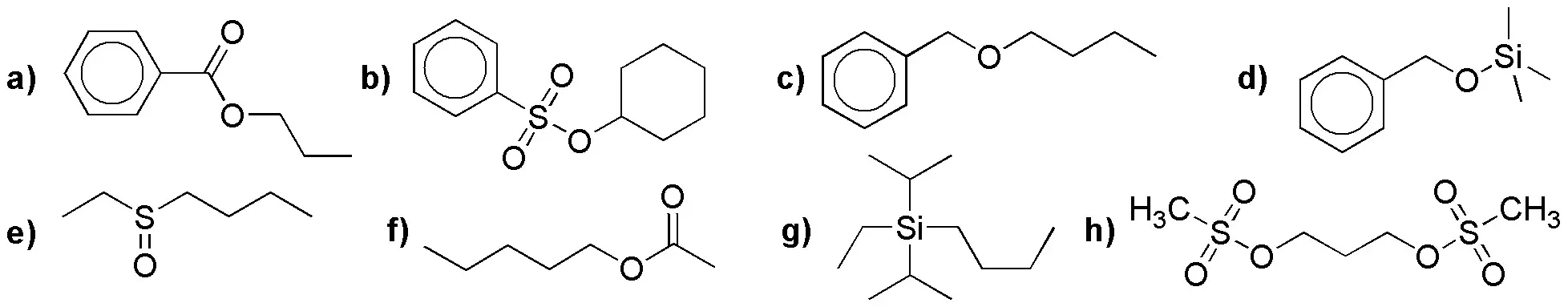
Problem 22)
Complete the following reactions:
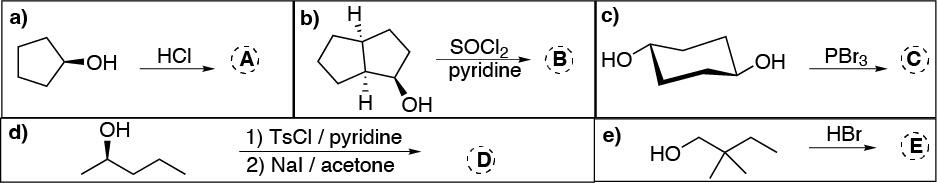
Problem 23)
Draw the structure of the alkene that mostly forms in the dehydration reaction of the following alcohols:
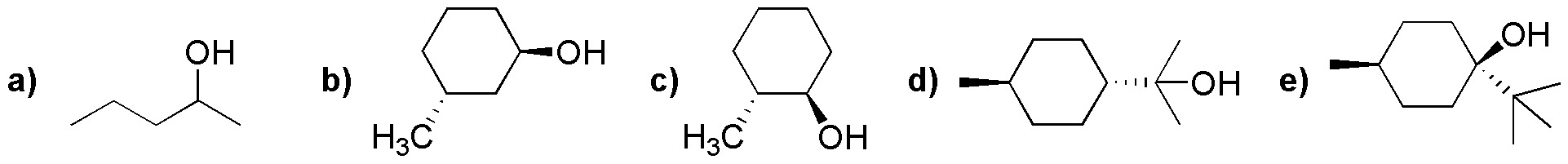
Problem 24)
Draw the most probable structure of the starting alcohol that by treatment with phosphoric acid produces the following alkenes:
![]()
Problem 25)
Describe what will be the major product of 1-phenylpropan-2-ol with the following reagents.
- a) SOCl2/Et3N
- b) Na then CH3I
- c) PCC / CH2Cl2
Problem 26)
When 2-cyclobutylpropan-2-ol is treated with HBr mostly 2-bromo-1,1-dimethylcyclopentane is obtained. Justify in a reasoned way the result.
Problem 27)
Complete (A-L) in the following table:
| Compound | PCC | Jones’ Reagent | Swern’s Reagent | KMnO4 |
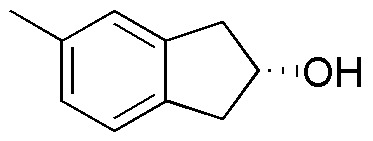 | A | B | C | D |
| E | F | G | H | |
| I | J | K | L |
Problem 28)
Indicate in each case, the reagents (A-F) needed to perform the following transformations:
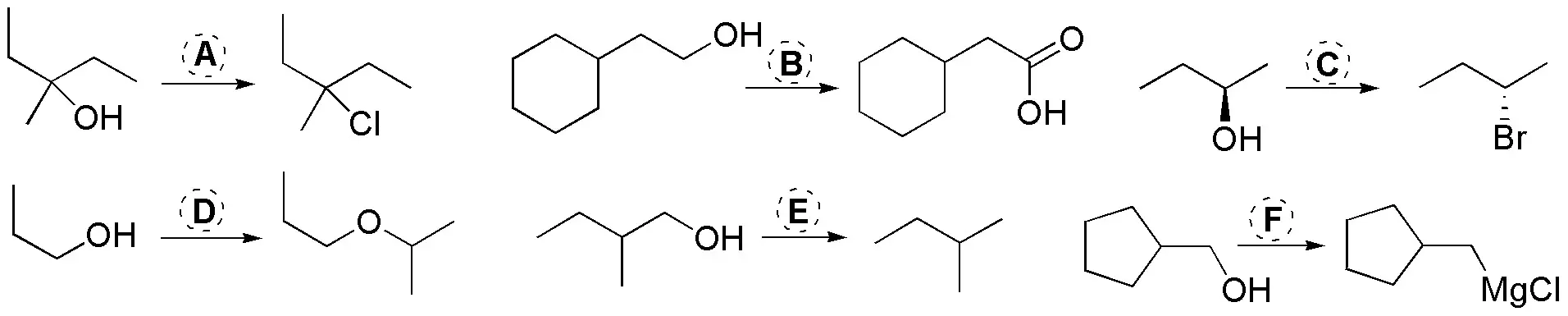
Problem 29)
a) Deduce in a reasoned way the result of the treatment of the following ethers (I-IV) with 1 mol of HI.
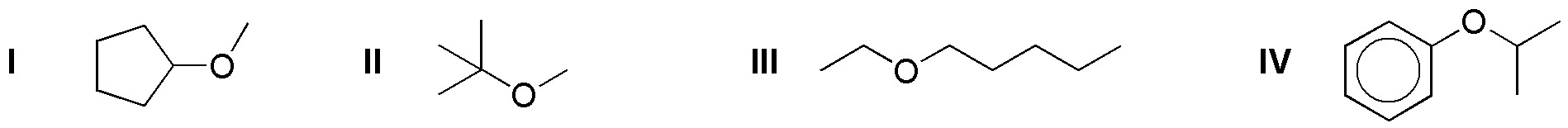
b) Indicate the result when an excess of HI is used.
Problem 30)
Complete the following reactions:
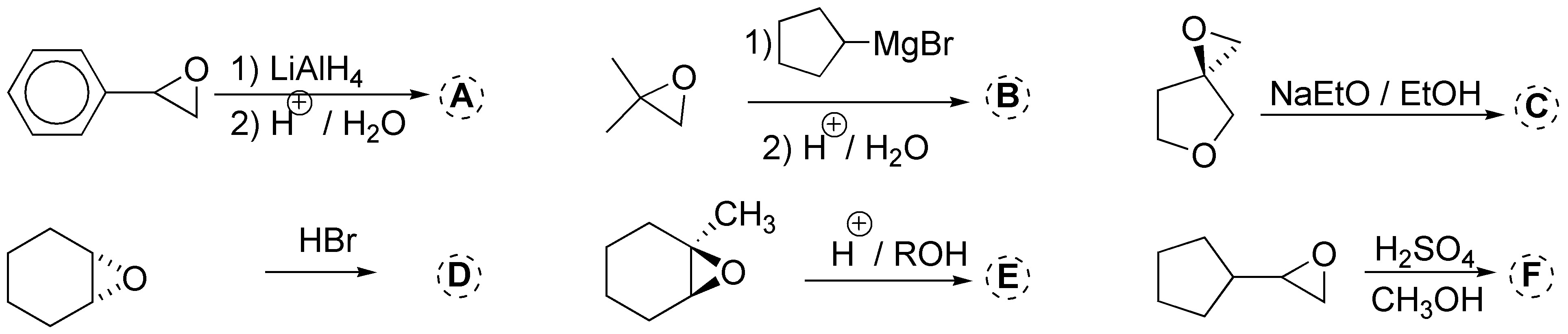
Problem 31)
Choose the reagents needed to perform the following transformations:
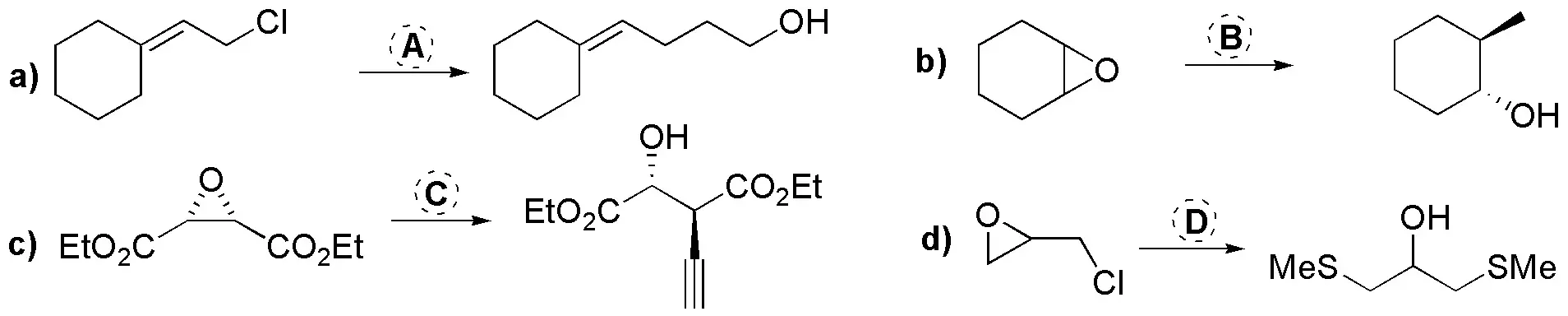
Problem 32)
Explain in a reasoned manner why the following transformations occur:
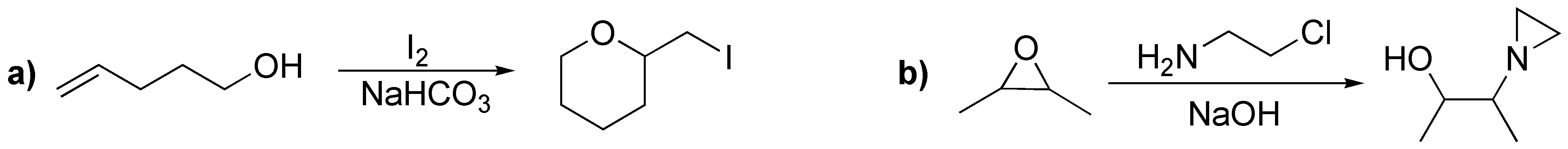
Problem 33)
Describe the result of each of these reactions, specifying whether the products obtained (A-S) are racemic mixtures, diasteromers or achiral molecules.
| Substrate | 1) LiAlH4 ; 2) H3O+ | (Et)2CuLi | H2O / H3PO4 | CH3CH2OH / H3PO4 |
|---|---|---|---|---|
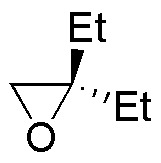 | A | B | C | D |
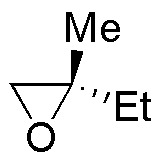 | E | F | G | H |
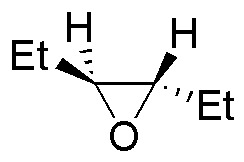 | I | J | K | L |
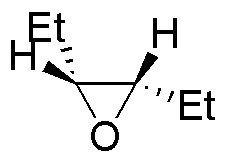 | M | N | O | P |
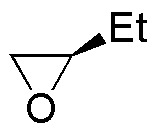 | Q | R | S | T |
Problem 34)
Complete the following scheme:
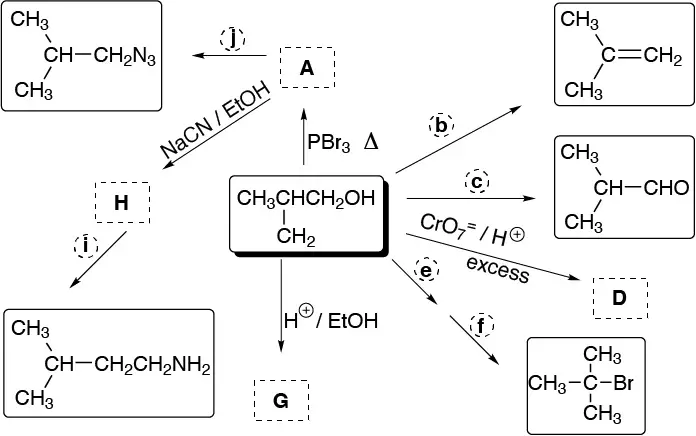
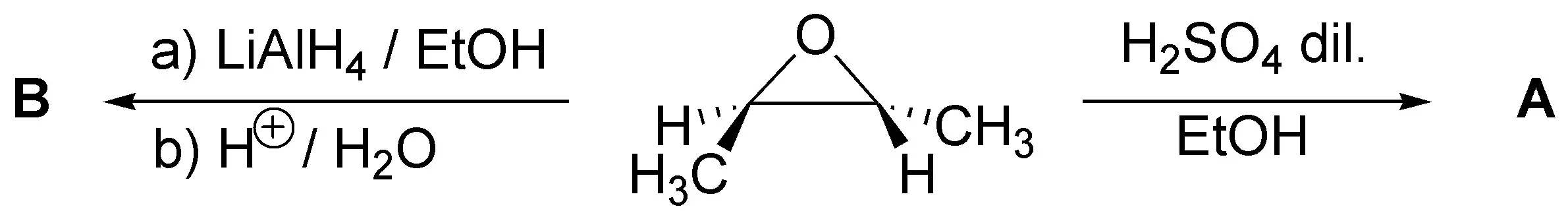
Problem 35)
Complete the following scheme:
Problem 36)
State what would be the major product of the reaction of 3-methylpentan-2-ol with the following reagents and conditions:
(a) NaH; (b) PBr3; (c) concentrated HBr; (d) thionyl chloride; (e) H2SO4 conc. at 130°C; (f) H2SO4 diluted in tert-butanol.
Problem 37)
Indicate what would be the major product of the reaction of 1,1-dimethyloxycyclopropane with the following reagents and conditions:
(a) H2SO4 diluted in MeOH; (b) NaMeO / MeOH; (c) dilute HBr; (d) concentrated HBr.