What are microscale techniques?
Organic Chemistry is an experimental science and this means that laboratory work is essential for the teaching of the subject. It is therefore important that the student who starts studying chemistry acquires the necessary knowledge to be able to work with ease and good habits in a laboratory or in a later professional activity.
The work in an organic chemistry laboratory can be carried out at three levels, depending on the amounts of substance handled: macroscale (from 5 to 50 g. of reagents and > 50 ml of solvent), miniscale (from 1 to 5 g. of reagents and about 25 ml of solvent) and microscale (< 1 g. of reagents and about 5 ml of solvent).
The current trend in the performance of laboratory practices is to use the mini-scale. However, the development of more sophisticated experiments and the use of more expensive products makes that in many cases it is advisable to minimize the expenses in the realization of the same, so there is a tendency to reduce the scale of the experiments, on the other hand also the microscale is the most usual way of realization of experiments at research level.
The biggest challenge of this scale is the adaptation of the laboratory material, as well as the adaptation of the basic operations to be performed.
Scales in the Organic Chemistry Laboratory
The work in an organic chemistry laboratory can be carried out at three levels, depending on the quantities of substances to be handled: macroscale, miniscale and microscale.
Macroscale
For a long time, laboratory experiments have been carried out using quantities of reagents in the order of 5 to 50 g and solvent volumes between 25 and 500 ml, known as macroscale or multigram scale techniques.
Nowadays, there is a tendency to drastically reduce the quantities of substances handled, so this methodology is being progressively shifted towards teaching experimental organic chemistry with much smaller quantities of substances.
However, considerable quantities of products are still prepared in research laboratories when the need for starting material arises, either for the development of a specific project or for the scaling up of a process towards the industrial synthesis of that substance.
It must be taken into account that the problems involved in the industrial synthesis of any product are very different from the techniques used in research laboratories (agitation, heating, purification, etc.), so that an adaptation of the laboratory processes to the industrial ones is required.
Miniscale
Most of the practices in organic chemistry are nowadays carried out at this scale. This methodology allows the isolation and characterization of the products using conventional laboratory material and allows to have very manageable quantities of products obtained. As main characteristics of these techniques we can highlight:
- Reagents: Reagent quantities between 1 and 5 g are generally used. At this scale, balances, for example, with a tenth of a gram of precision can be used and the errors made will not be significant.
- Solvents: The volumes of solvents used for both reactions and extraction processes are usually around 25 ml. The amount of solvent is usually not critical for the performance of the experiment. For example, if 20 ml of diethyl ether is needed for a reaction, the reaction will probably proceed with the same yield if 18 ml or 25 ml is used. For the measurement of the volumes, a 25 ml graduated cylinder or a 10 ml pipette will be sufficient, but this does not mean that it is not necessary to be accurate in the measurements.
- Material: The material used will be the conventional material of an Organic Chemistry Laboratory. It will only be necessary to adapt it to the volume of solvent or amount of reagents used in each specific experiment.
Microscale
Research in laboratories is usually carried out on this scale. In recent years, there is a growing tendency to apply this methodology in the teaching of experimental organic chemistry, especially with students who are already initiated.
- Reagents: The experiments carried out in microscale organic chemistry are carried out with amounts of the main reagent between 0.005 and 0.5 g. Weighing should be carried out on balances with at least two decimal places, preferably with three decimal places. Note that with these quantities, a deviation of 0.1 g in a reagent is a very significant percentage error in the proper proportions of the reagents used.
- Solvents: Solvent quantities are usually below 100 microliters and 5 milliliters. Therefore, pipettes, micropipettes, dispensers or syringes with the appropriate graduation and precision for each experiment should be used.
- Material: The material used in microscale requires adaptation to the quantities used, especially when these are less than 100 mg. This material can have different configurations ranging from material similar to conventional material, only with a size adapted to the needs of the quantities and volumes used in these techniques, or specifically designed material (“micro-scale kits“).
Advantages of microscale work
The advantages of using microscale techniques in organic chemistry laboratories are very evident. Among the most relevant we can mention:
- Reduces the costs of teaching practices per student in each experiment.
- It makes it possible to increase the number and repertoire of practice experiments with the same budget.
- It allows experiments involving the use of more expensive reagents.
- Improves safety in the laboratory by reducing exposure to potentially toxic substances and risks of explosion or fire.
- It significantly reduces the amount of reagents used and consequently the waste generated.
- It usually involves less time for reaction and experimentation, so more time can be spent analyzing the results.
- Improves the use of laboratories.
- It allows the development of new techniques for the use of laboratory equipment.
- It requires less storage space for reagents and materials.
- It promotes the principle of the three Rs: Reduce, Recycle, Recovery.
- Improves the training of students, as it forces them to be more careful at all stages of their internship work.
Specific microscale material
There are different specific microscale equipment on the market, made up of material designed for this purpose, which allow most of the basic laboratory operations to be carried out. There are two configurations that derive from the publication of two books dedicated to the description of experiments.
Kontes-Williamson equipment
These kits were designed by K.L. Williamson and initially manufactured by “Kontes Glass Co.“, as described in the book Macroscale and Microscale Organic Experiments. It is a relatively inexpensive and fairly simple “kit“, where elements similar to conventional material but of a reduced size are combined.
The “kit” consists of: Erlenmeyer flasks, Kitasatos, centrifuge tubes or Hirsch funnels, among others. They are specifically designed as: flasks with different types of neck, distillation heads and columns, reaction tubes or chromatography columns in which the frosted joints, which make the price more expensive, have been eliminated and replaced originally by flexible connectors.
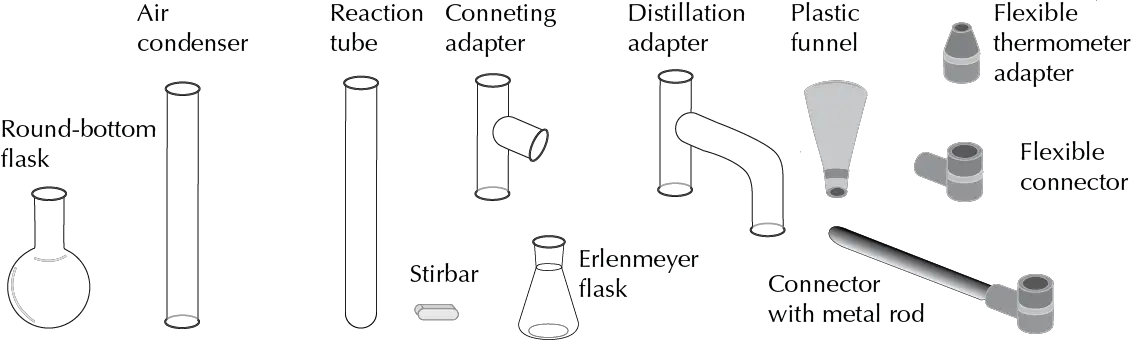
Recently, however, such equipment has become available with the incorporation of threaded plug-based joints.
ACE-Mayo equipment
They have become the most popular and versatile kits. The name comes from the company that first developed such “kits” (“ACE Glass Inc.“) following the appearance of the book Microscale Organic Laboratory, (Mayo, Pike and Butcher) in 1986.
In this equipment, the different parts are connected by means of ground and threaded joints. The use of coolant grease is discouraged to avoid contamination of samples and reactions, so O-rings are used to ensure the tightness of the assemblies.
The Figures illustrate the main elements of such equipment:
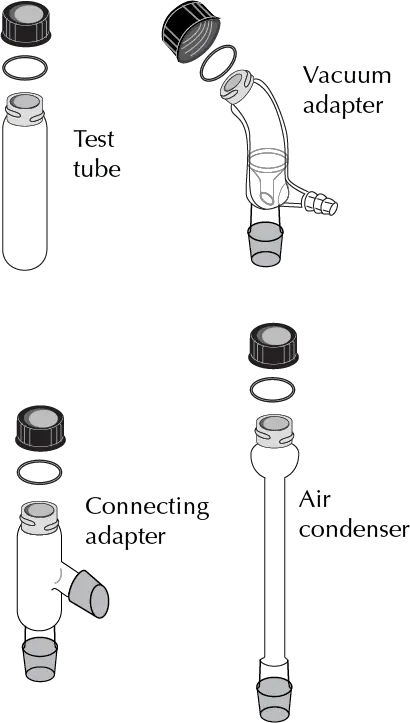
The most significant differences between the components of this equipment and the one normally used in practice (macroscale and miniscale) are the following:
- Conical vials: The preferred use of conical vials is widespread both for carrying out reactions and for liquid-liquid extractions. This form is especially effective for liquid handling so that with a disposable pipette one of these vials can be completely emptied.
- Triangular stirring magnets: Also adapted for use in conical vials, they have a triangular shape that allows them to rotate at any speed with total efficiency.
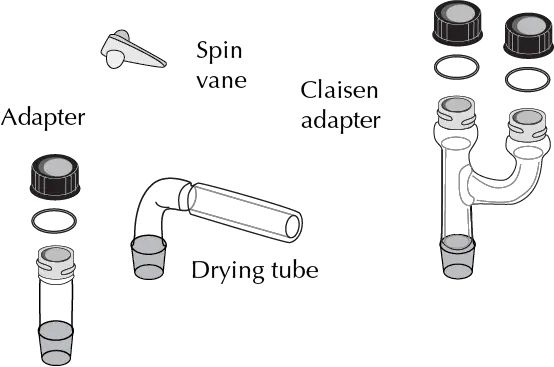
- Craig tube: Used for filtering in the recrystallization of solids.
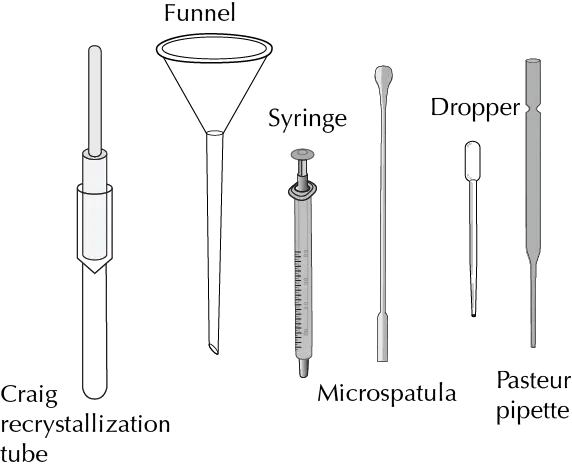
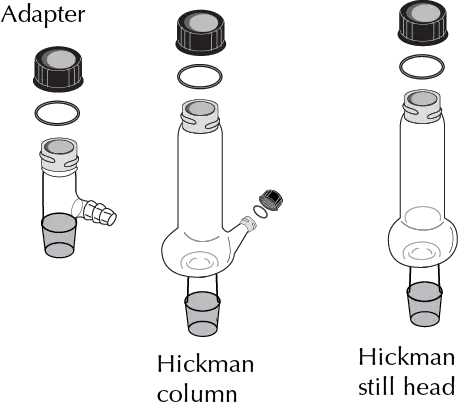
- Hickman head (or column): It replaces the traditional distillation set-up (Claisen adapter, straight coolant and manifold). It can have a side opening that allows easy removal of the distillate obtained with the aid of a Pasteur pipette.
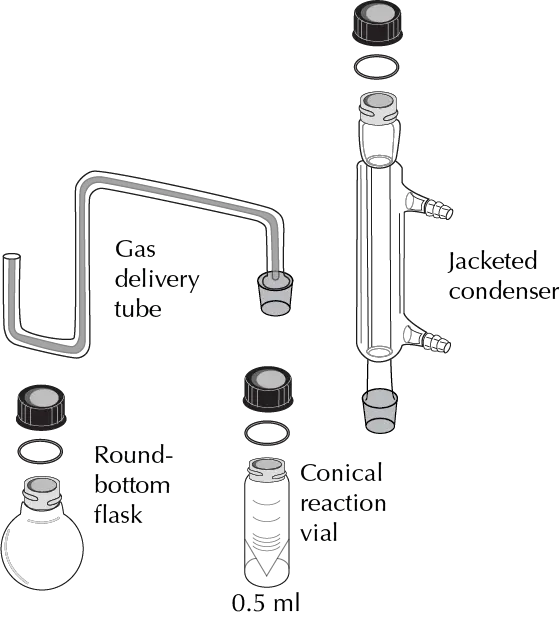
- Gas generator tube: Used to measure the amount of gas produced in a reaction or for the collection of hazardous gases.
Basic operations and specific microscale techniques
Unlike conventional laboratory techniques, working on a much smaller scale of solid and liquid quantities for reagents and solvents involves adapting the basic laboratory operations listed in the following table:
| Mass and volume measurements | Removal of small amounts of solvents |
| Filtration | Liquid-liquid extraction in conical vials |
| Craig tube recrystallization | Drying liquids and solids |
| Sublimation | Reflux |
| Distillations | Reagents addition |
| Inert atmosphere reactions | Removal of harmful vapors |
| Column chromatography CC | |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- PYREX® Chemistry Kits and PYREX® Microscale Organic Chemistry Kits, Corning