What are Lewis structures?
Gilbert Newton Lewis proposed that for a molecule to be stable its valence shell should be complete. Hydrogen, with 1 electron in an s orbital, needs only 1 electron to complete its valence shell. However, since carbon has 4 valence electrons, it will need to complete the 4 valence (s , px, py, pz) orbitals with 4 additional electrons, to reach a total of 8 valence electrons (octet rule). This electronic configuration is particularly stable and similar to that of a noble gas.
To visualize this, G.N. Lewis developed a representation system where valence electrons are indicated around atoms with dots (–) and a pair of electrons with a colon (:). It is still considered one of the most famous forms of structure and representation of organic molecules.
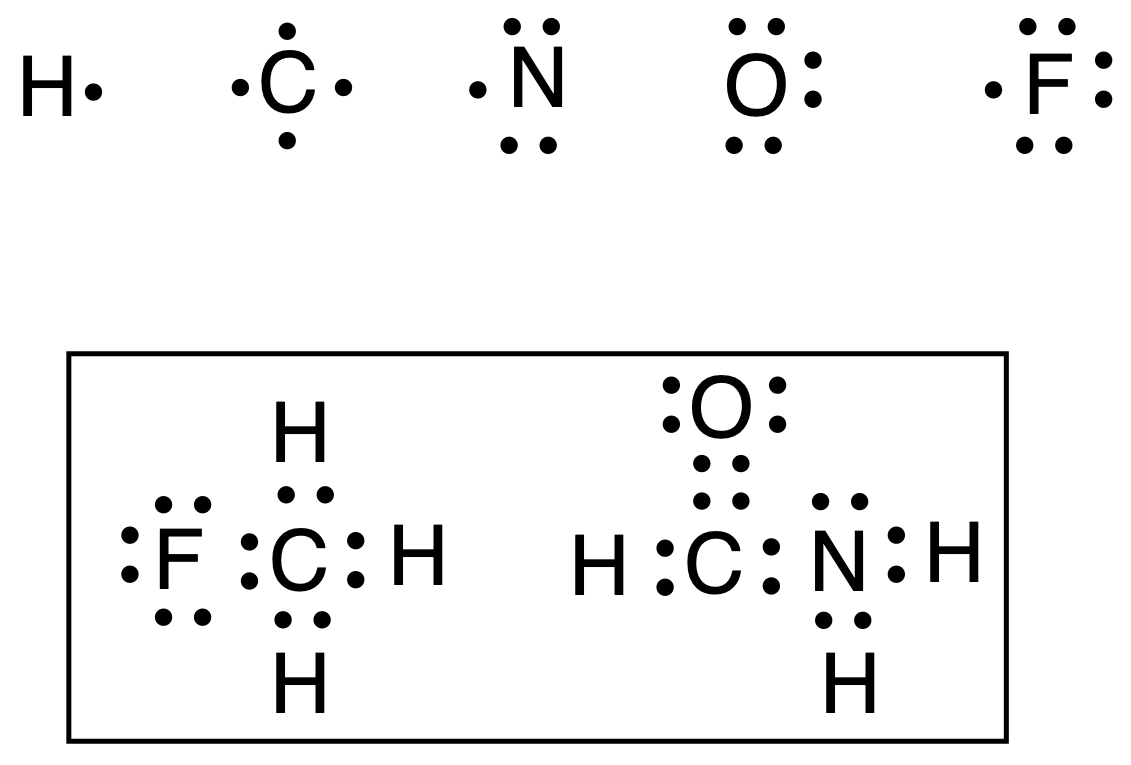
For example, in the figure above, hydrogen, carbon, nitrogen, oxygen and fluorine atoms are depicted with their valence electrons (1, 4, 5, 5, 6 and 7, respectively), as well as Lewis structures for CH3F (fluoromethane) and HCONH2(formamide) molecules. Alternatively, the electrons can be represented with the symbols to visualize more clearly their «origin«.
In addition, to further simplify the structures, instead of dots, lines (–) are drawn to represent bonds and dots for non-bonding electrons (non-bonding electrons). In other texts such representations appear with lines only.
The following figure shows different forms of Lewis representation for the ClCCONH2 molecule. Regardless of how the electrons are drawn, they all show that oxygen has two unshared electron pairs and forms a double bond with carbon, nitrogen has only one unshared electron pair, and chlorine has three.
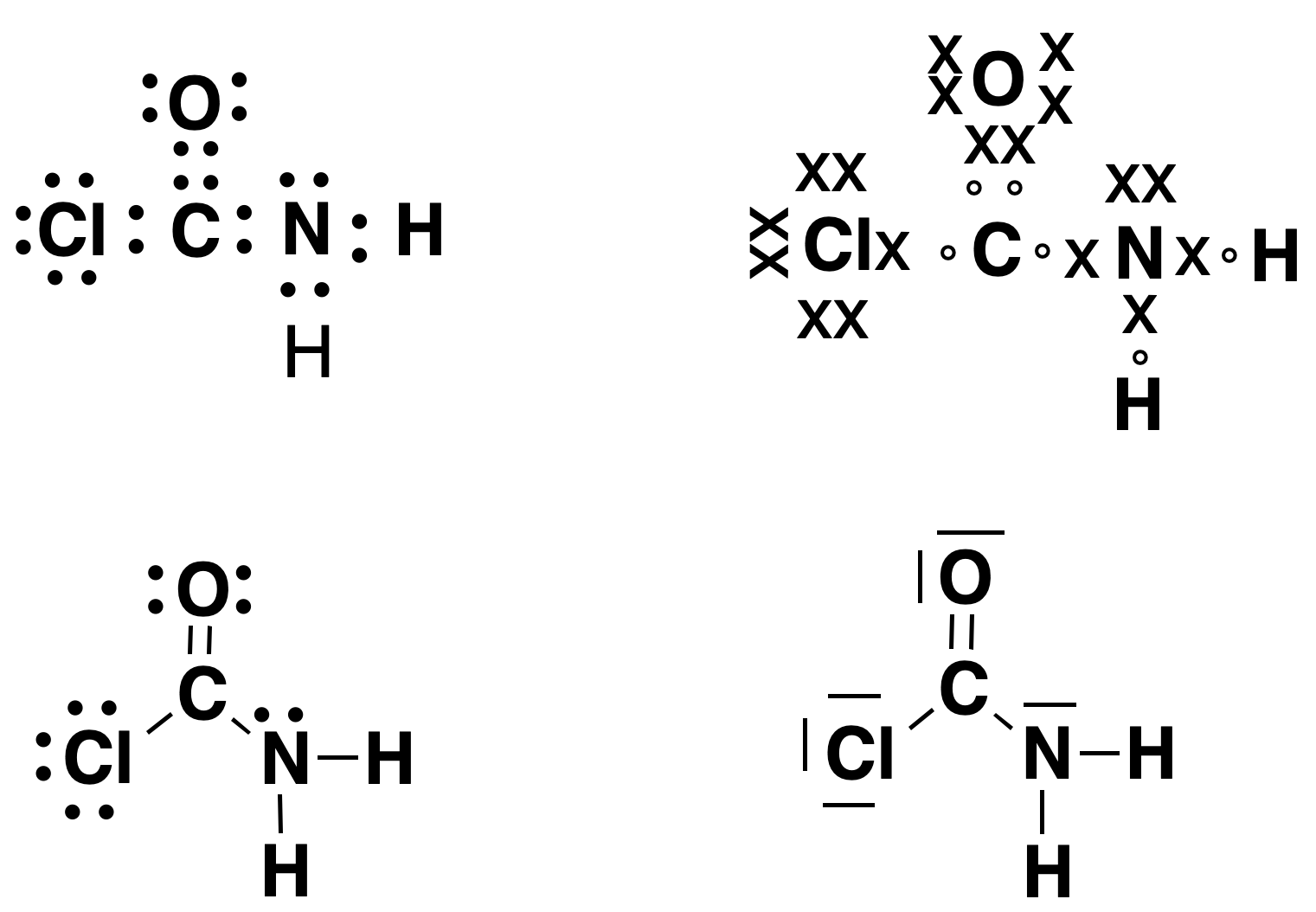
To summarize, Lewis structures show, in a simple graphical form, the number and type of bonds in a molecule (connectivity), as well as the number of unshared electron pairs, or unpaired electrons.
Formal charge
Formal charge is a concept in chemistry that is used to determine the distribution of electrons in a molecule or ion. It is a way of assigning charges to individual atoms in a molecule or ion, based on the number of valence electrons it has, and the number of electrons it shares with other atoms in the molecule.
Thus, the formal charge of an atom is the charge that atom would have if all the bonds present in that atom were pure covalent.
The formal charge on an atom is calculated by the following formula:
formal charge of an atom = (no. e– valence) – (no. e– non-bonded) – ½ (no. e– bonded)
For example, the formal charge of the carbon atom is 0 in methane, and we calculate it as follows:
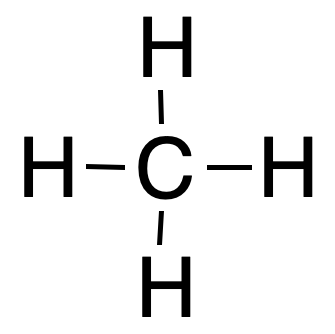
The number of valence electrons of carbon is 4.
It has no unbonded electrons on carbon.
The bonded electrons of carbon are 8.
Therefore, the formal charge of carbon will be zero = (4) – (0) – ½(8) = 0.
Resonance structures
Within the structure and representation of organic molecules, there are many that have particular properties due to a special electron density distribution. Among these are the aromatic compounds, for example benzene. Such compounds can be described by various Lewis structures, and are called resonant structures, and differ only in the distribution of their electrons (not that of the atoms). For example benzene can be represented, mainly, by two resonant structures shown in the figure.
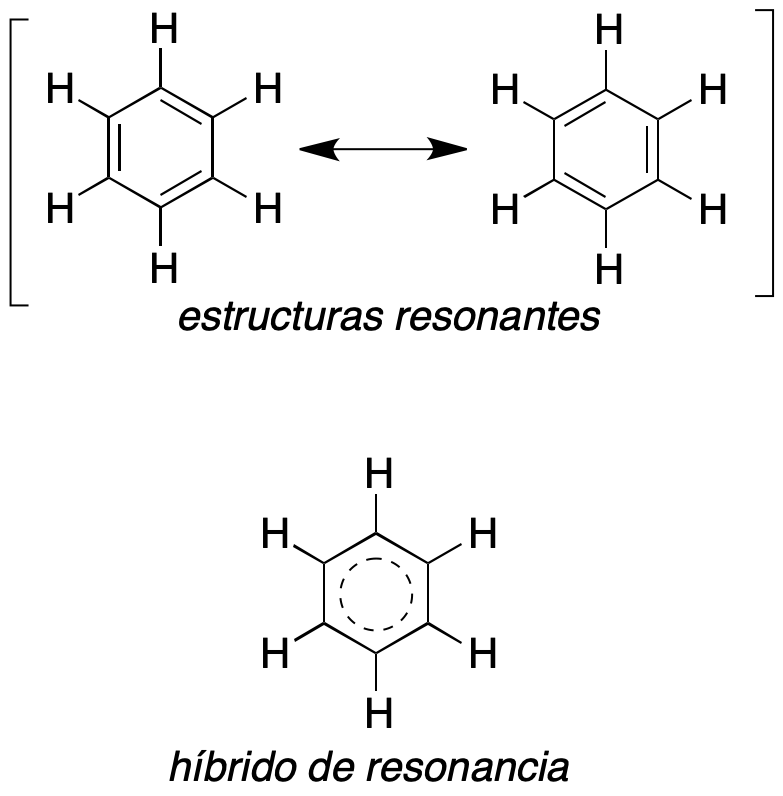
It should be noted that both describe benzene, being in reality a structure with intermediate characteristics (for example, the length of the C-C bonds would be intermediate between that of a single and a double bond). Resonant structures are usually indicated in parentheses and are related with a double-headed arrow, which should not be confused with systems in equilibrium, reactions, etc.
Furthermore, we should not change the nuclear positions and orientation of the molecule in such structures. The resonance hybrid would be a unique structure representing this situation.
The different resonant structures have a greater or lesser contribution to the resonance hybrid depending on the stability of each one of them (structures with lower energy contribute more), and such contribution can be qualitatively evaluated taking into account the following considerations:
a) The most stable resonant structure (that contributes the most) is the one with the highest number of covalent bonds.
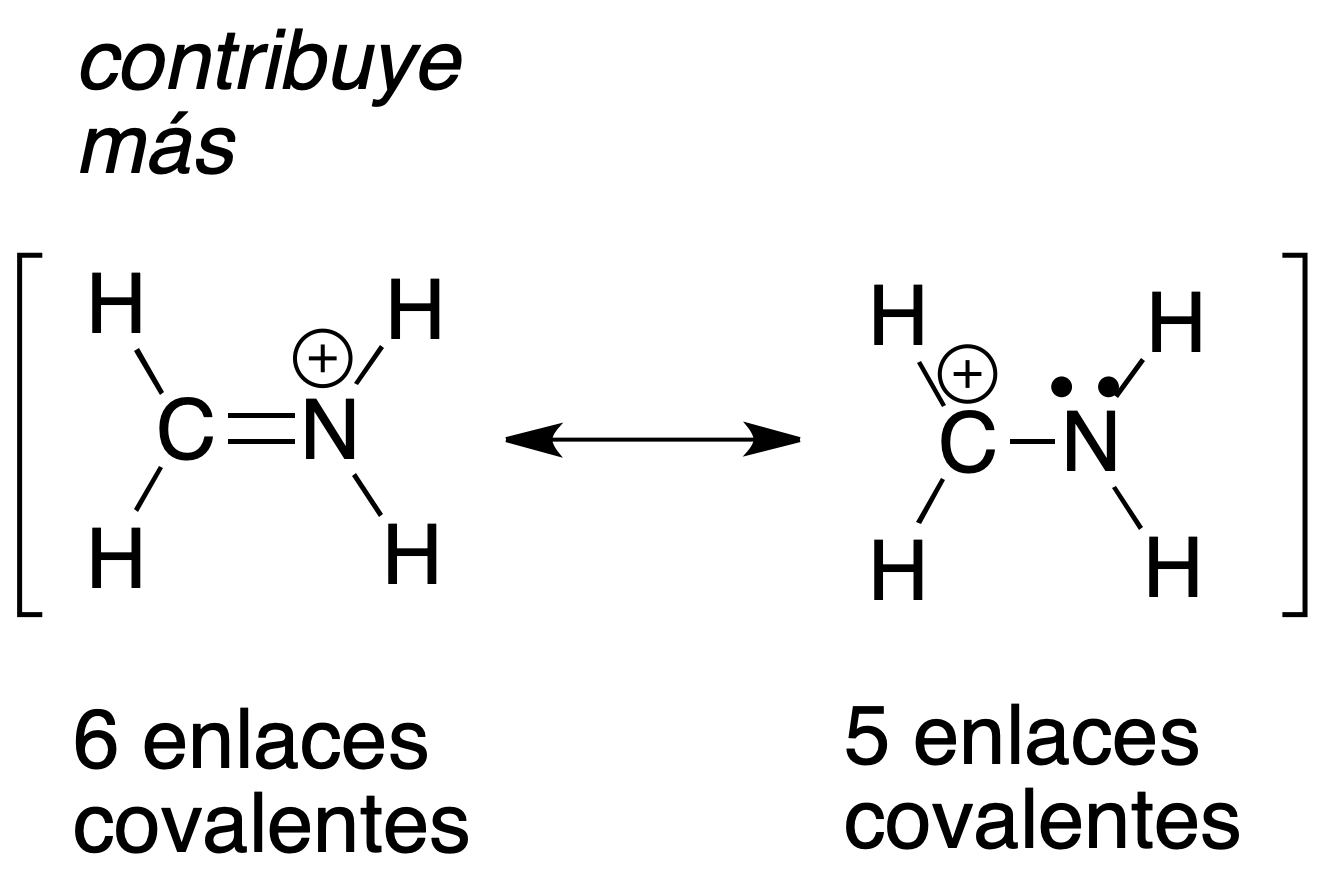
b) The most stable resonant structure (contributing more) is the one with the highest number of octets (2 and 1 in the previous case).
c) Uncharged structures are more stable (contribute more) than those with charge separation.
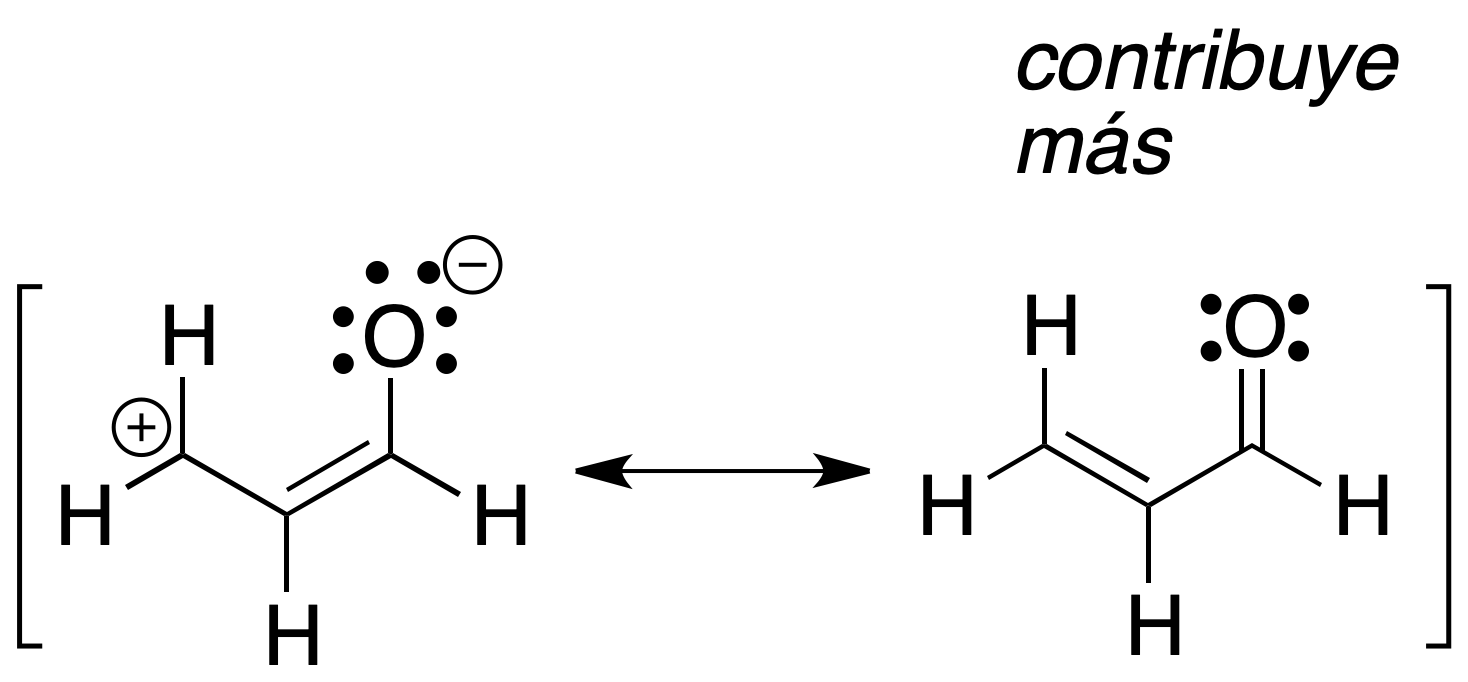
d) When comparing two resonant structures with charge, the one with its negative charge on the more electronegative atom will be more stable.
e) When comparing two resonant structures with charge, the one with a minimum separation of charges of different sign will be more stable. Also, the structure with the same charge on adjacent atoms is more unstable.

Examples of Lewis structures
The Lewis structures can provide valuable insights into the bonding and electron distribution within a molecule. Here are a few examples of Lewis structures for different molecules.
- The Lewis structure for carbon dioxide (CO2) consists of one carbon atom bonded to two oxygen atoms, with no lone pairs of electrons.
- The Lewis structure for methane (CH4) consists of one carbon atom bonded to four hydrogen atoms, with no lone pairs of electrons.
- The Lewis structure for ammonia (NH3) consists of one nitrogen atom bonded to three hydrogen atoms, with one lone pair of electrons on the nitrogen atom.
Other examples:
- Lewis structure of borane BH3
- Lewis structure of acetone CH3COCH3 molecule
- Lewis structure of nitrogen trifluoride NF3 molecule
- Lewis structure of nitrate ion NO3–
- Lewis structure of nitrogen trichloride NCl3
- Lewis structure of water H2O
- Lewis structure of hydrogen sulfide H2S
- Lewis structure of carbon monoxide CO
- Lewis structure of acetic acid CH3COOH (ethanoic acid)
- Lewis structure of carbonate ion CO32-