Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the solutions to the problems.
Aldehydes and Ketones – problem list
Problem 1)
Identify the product obtained in each of the following reactions:
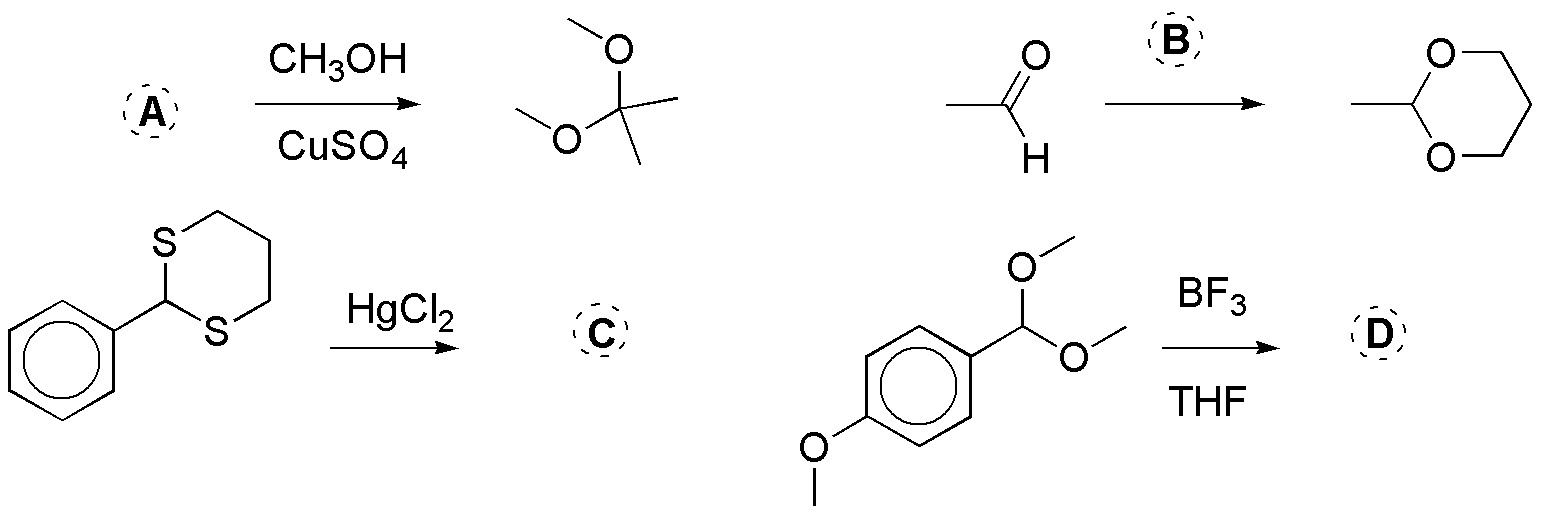
Problem 2)
Indicate the reagent needed to carry out the following transformations:
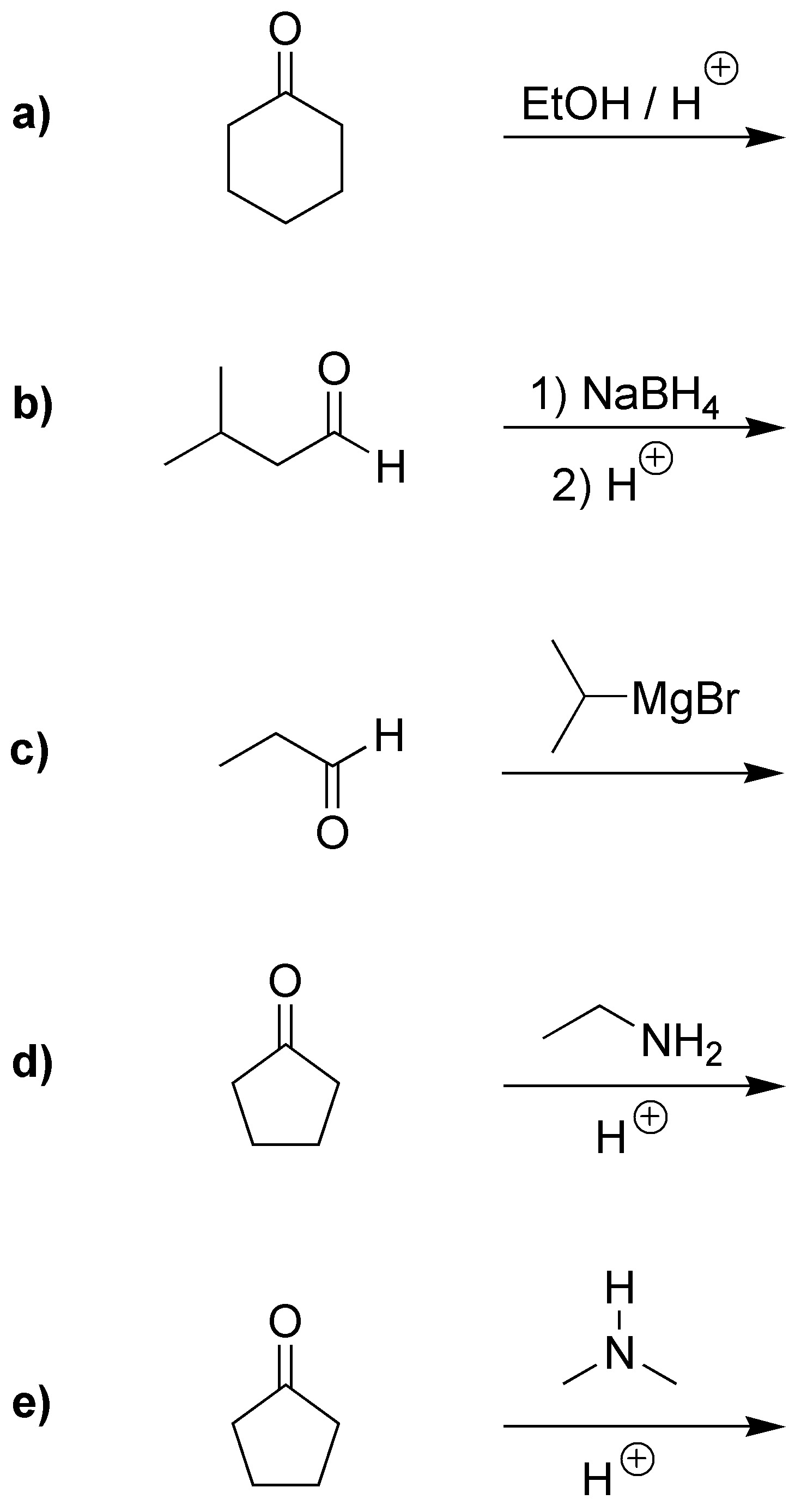
Problem 3)
Complete the following reactions:
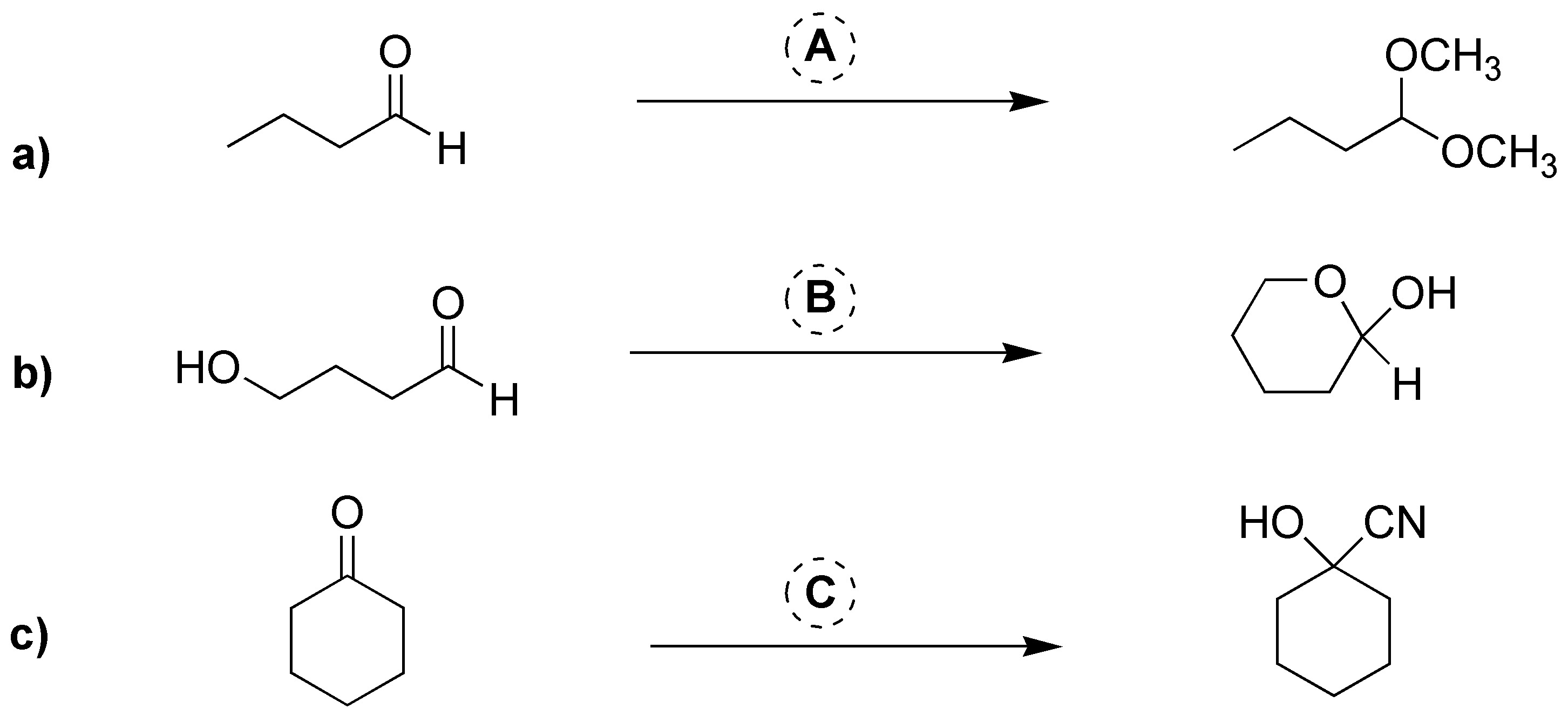
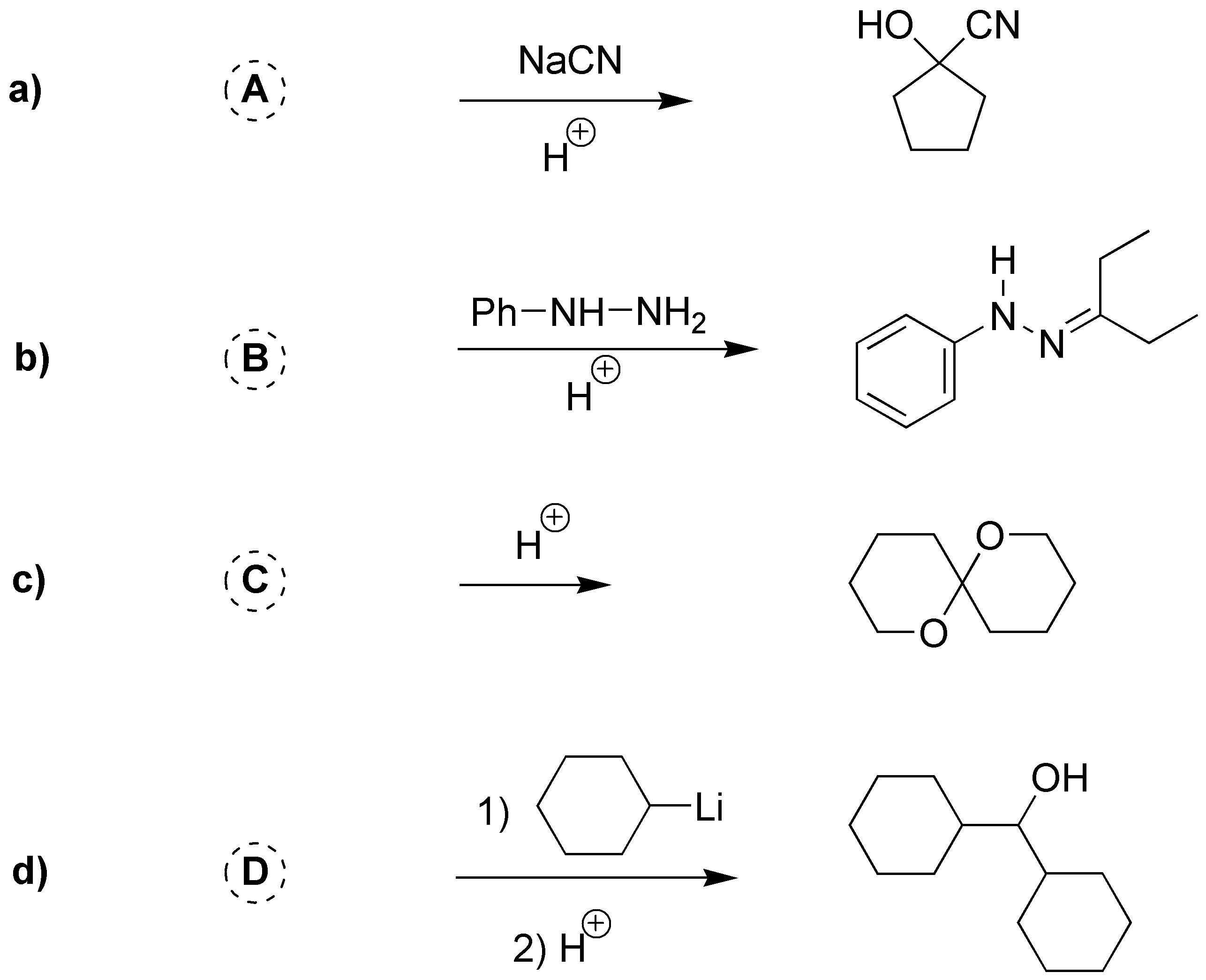
Problem 4)
Choose the appropriate carbonyl compound to obtain the following products:
Problem 5)
What products are obtained in the following oxidation reactions of aldehydes and ketones?

Problem 6)
Predict the major product in the reaction of phenylmethylketone (acetophenone), describing the intermediate formed, if we use as oxidizing agent the carboxylic peracetic acid MCPBA.
Problem 7)
Write the product(s) obtained by catalytically reducing with H2 and Pd(C) the following compounds:

Problem 8)
Write the product obtained, from the reduction of the following compounds, in a first step with LiAlH4 in THF and subsequent acid treatment in water (H3O+).

Problem 9)
Write the product obtained from the following reductions with NaBH4 in ethanol:

Problem 10)
What ilides and carbonyl compounds should you use in a Wittig reaction to obtain 2-methylpent-2-ene?
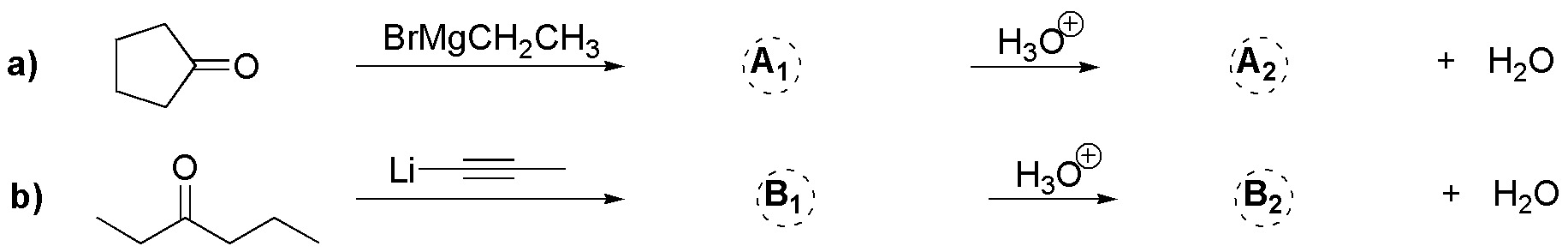
Problem 11)
Write the intermediate and product obtained, from the addition with Grignard and organolithium reagents of the following ketones:
Problem 12)
Explain in a reasoned way why strongly acidic conditions, which would favor the reactivity of the carbonyl group, cannot be used in these reactions.
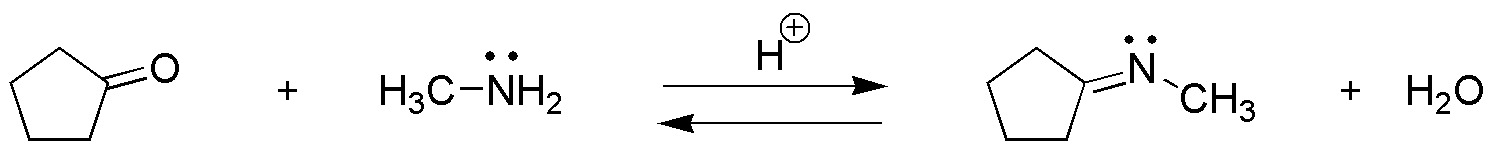
Problem 13)
Complete the following sequence of reactions:
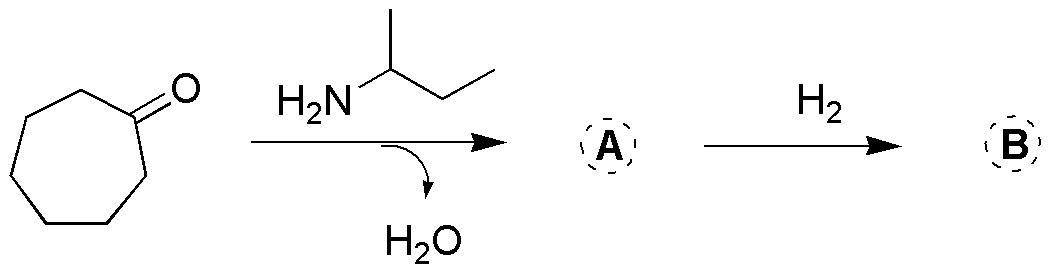
Problem 14)
Identify the compound in the following structures (I-IV), indicating a procedure for its synthesis, as well as how to regenerate the starting compound.
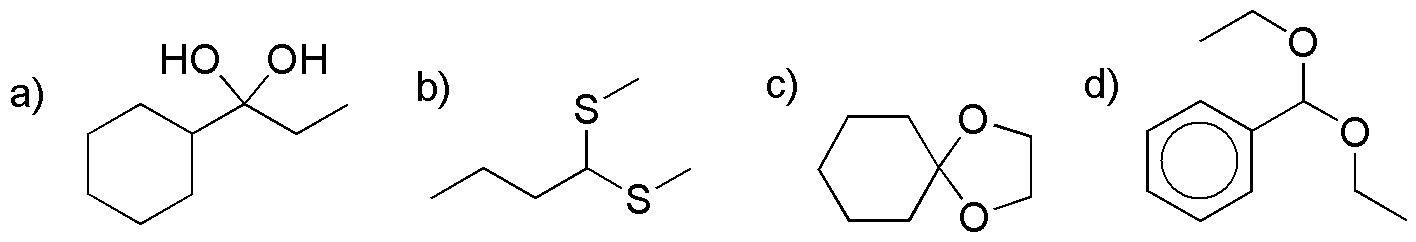
Problem 15)
Complete the following reaction scheme:
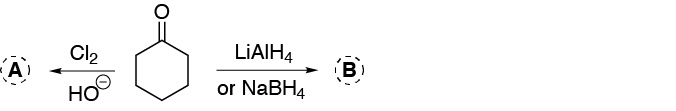
Problem 16)
Predict the major product in the reaction of the following compounds with MCPBA:
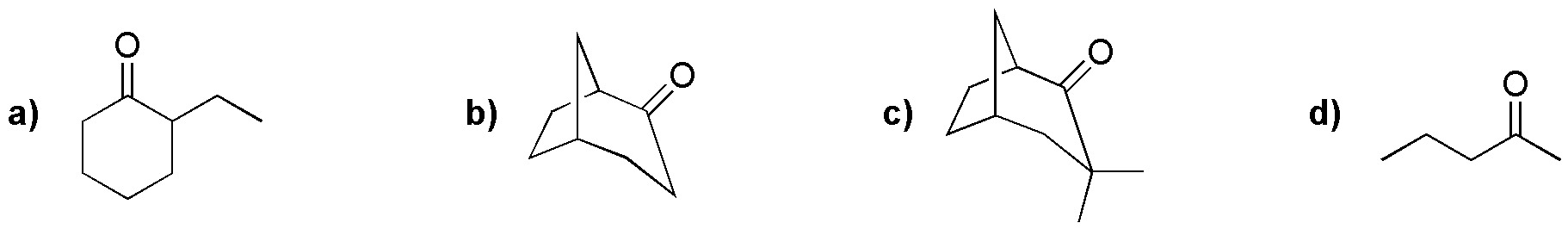
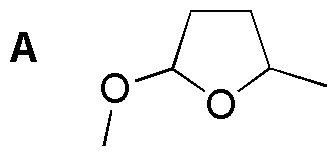
Problem 17)
Explain the formation of A from 4-hydroxypentanal when treated with 1 mol of methanol.
Problem 18)
Complete the following scheme:
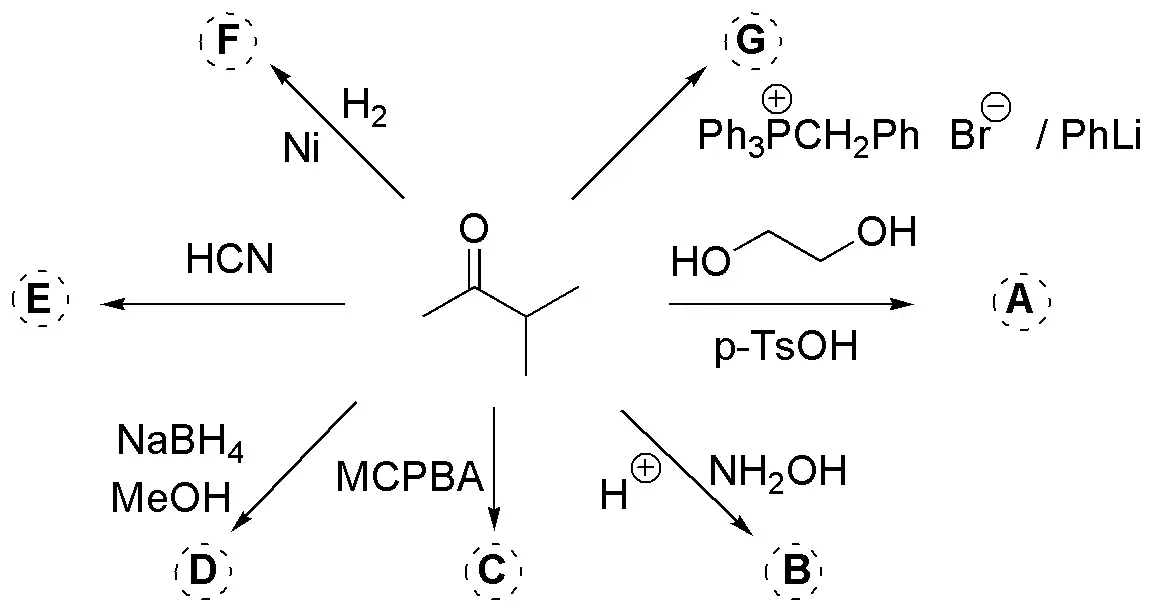
Problem 19)
Complete the following reactions:
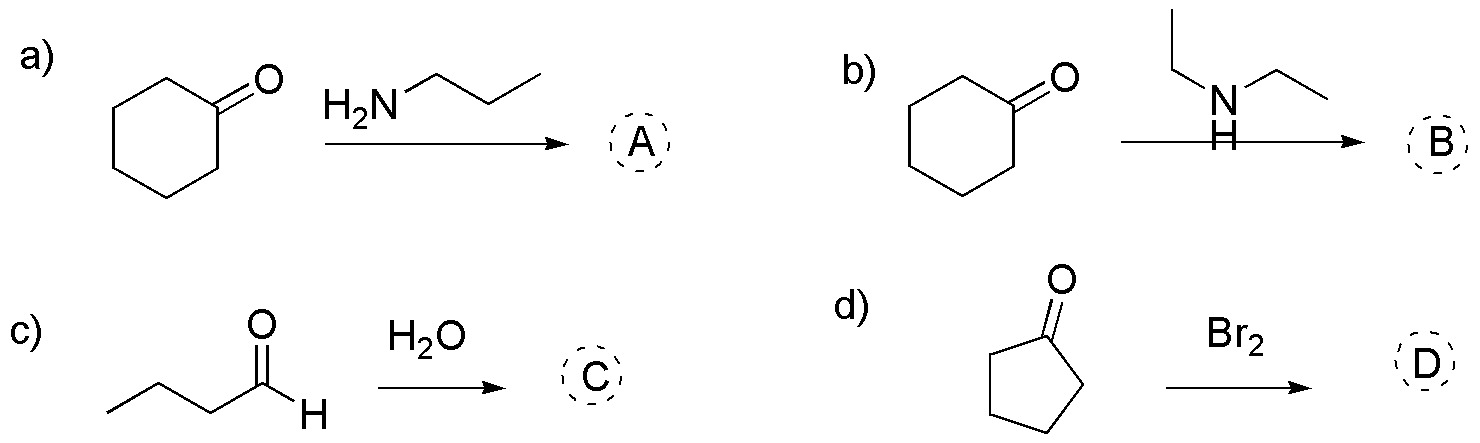
Problem 20)
Propose a procedure for the preparation of the following alcohols using cyclopentanone as the starting compound:
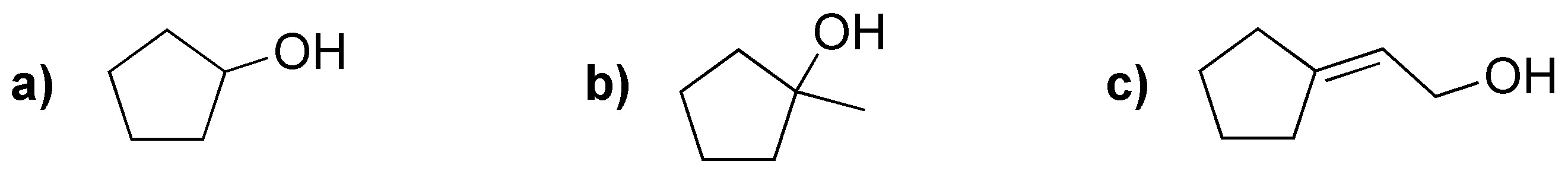
Problem 21)
Choose from the following substrates which ones would give the Cannizzaro reaction and draw the products obtained in each of them.
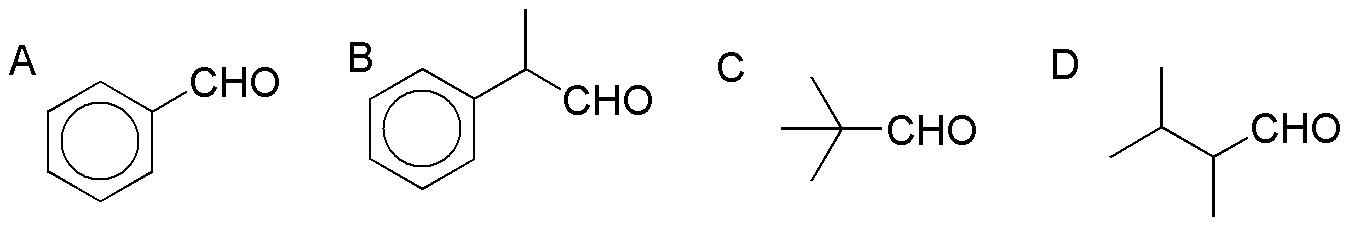
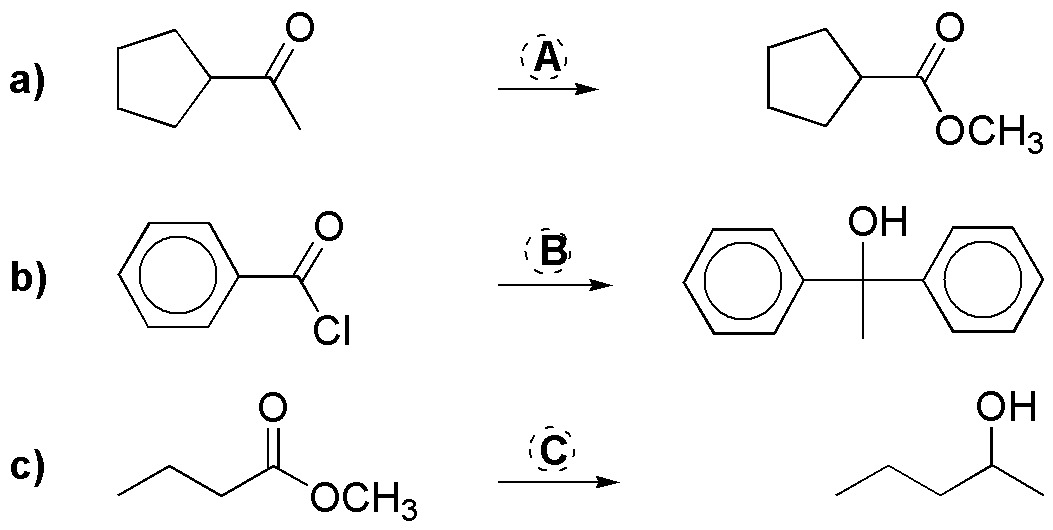
Problem 22)
Complete the following reactions (a-c):
Problem 23)
Indicate the result of treating benzaldehyde with the following reactants:
- a) pentane-2,4-dione / piperidine.
- b) 1) malononitrile / triethylamine; 2) H3O+ / Δ
- c) 1) nitromethane / dimethylaminopyridine; 2) LiAlH4 / THF
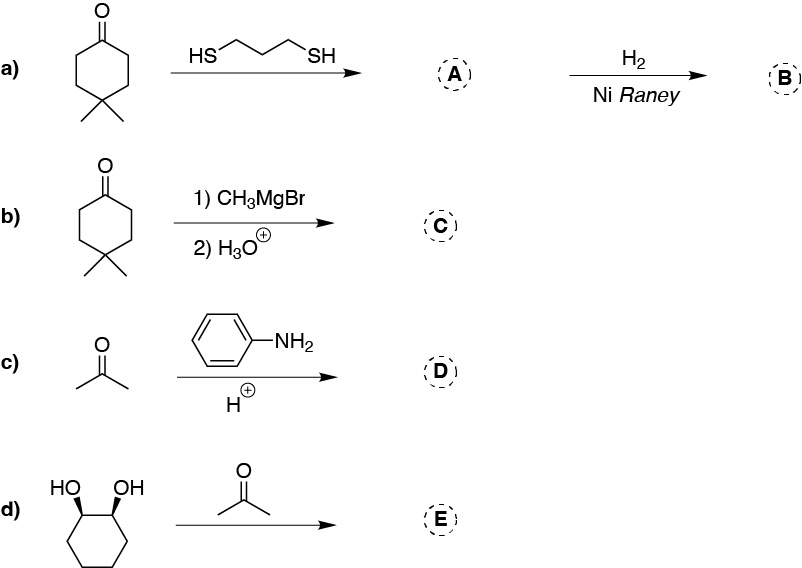
Problem 24)
Complete the following reactions (A-D):