Written by J.A Dobado | Last Updated on April 22, 2024
What is functinal-group analysis?
The qualitative organic analysis (or functional-group analysis) has a great relevance in the teaching of organic chemistry mainly due to its great formative value.
Unlike inorganic analysis, which identifies the constituent elements of a substance, organic analysis determines the functional groups, since most organic compounds are made up of only a few elements and are almost always the same.
That is why to identify organic compounds it is not useful to follow an analytical pathway parallel to the inorganic one, but we have to rely on the different chemical properties of the different organic functional groups together with the physical and spectroscopic properties of the product to arrive at the final identification of the problem substance.
Although at first sight it may seem extremely complicated to identify an organic compound, this identification can be approached by means of a well-defined systematic approach. The tactic to be employed consists, essentially, in a successive and progressive limitation of the structural possibilities.
This generally begins with the identification of the constituent elements of the product, followed by the identification of the functional groups present in the molecule through the use of solubility tests, the observation of its organoleptic properties and chemical tests.
Having determined the functions present and by preparing some derivatives of the product, we were able to unequivocally identify the problem substance by comparing the observations made with those reported in the literature.
Organic qualitative elemental analysis
When faced with an unknown organic substance, the identification work must begin by performing a qualitative elemental analysis (and quantitative if possible) to determine the elements present in the substance and the proportion in which they are present.
Quantitative analysis normally consists of the combustion of a certain weight of the substance, identifying chromatographically (or gravimetrically) the quantity and nature of the gases formed in such combustion.
As for qualitative analysis, the most common (and/or simple) method is the so-called Lassaigne method or Lassaigne test or sodium fusion, which consists of the mineralization of the organic substance by transforming the elements present in it into easily identifiable anions:
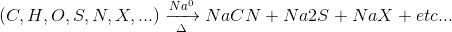
The complete procedure is detailed in the following link (Lassaigne’s test).
Organoleptic and physical properties
Once the qualitative elemental analysis has been carried out and the elements present in it have been determined, we should note the appearance of the substance (organoleptic properties): state, color, odor, crystalline form (if solid), ignition test (if it burns completely or leaves a solid residue, type of flame, etc.) and any other property that may give us additional information about the substance.
Next, we will determine the main physical constants, namely: melting point for solids and boiling point for liquids.
This will give us an idea of the degree of purity of the substance and will indicate the need for purification either by recrystallization (after solubility tests) or by simple or vacuum distillation, determination of the refractive index, density, rotational power (if optically active).
And finally we will obtain, if possible, spectroscopic data, mainly IR and NMR spectra.
Classification by solubility
On the basis of the different solubility of organic compounds in different solvents, these compounds can be classified into various groups. The phrase “like dissolves like” allows us to separate the most common types of functional groups.
Thus, nonionic compounds do not dissolve appreciably in water unless their molecules are ionized in aqueous solution or can associate with water molecules by hydrogen bonds.
Those lacking hydrogens capable of forming hydrogen bonds dissolve rapidly in ether and other unassociated solvents.
However, it should be noted that as one moves up in a homologous series, the solubility, like other physical properties, tends to approach those of hydrocarbons of the same number of carbon atoms.
Compounds with a basic character dissolve in acidic media and those with an acidic character dissolve in basic media.
By means of these simplified procedures we divide them into types and select functional groups. However, it should be noted that solubility tests are not infallible and exceptions are known.
Therefore, they will never be completely conclusive and we will have to perform other types of assays for the complete identification of a compound.
Procedure
The procedure to follow to carry out a qualitative organic analysis is the following: 0.2 ml (or 0.1 g if it is solid) of the problem is placed in a test tube. These are added with agitation and in portions 3 ml of water.
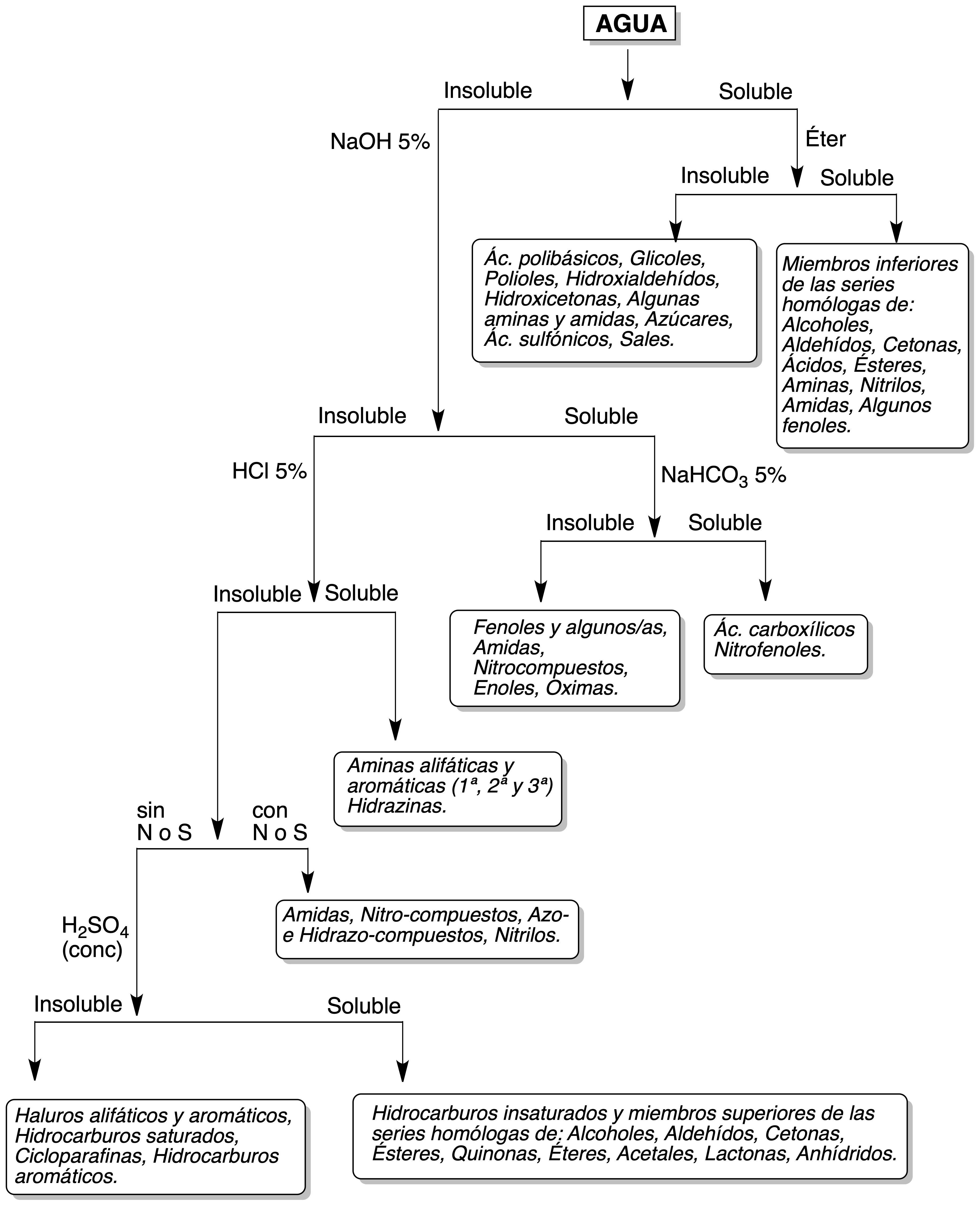
If the product is soluble, the process is repeated in another test tube in the same way but with ethyl ether as solvent.
If the compound is insoluble in water, its solubility is tested in a 5 % NaOH solution. All the compounds that are soluble in NaOH solution must be tested next with a 5% sodium bicarbonate solution.
These solubility tests are carried out in the same way as indicated for the water test. If the compound is insoluble in NaOH solution, its solubility is tested in 5% HCl.
Finally, if the test substance is insoluble in water, NaOH and HCl and if it contains neither nitrogen nor sulfur, its solubility in concentrated H2SO4 is tested.
In the Figure, the most important types of compounds are listed, classified according to their different solubility. This Figure is merely informative and is elaborated for substances relatively common in some practices of Organic Analysis Laboratory and that present the most common functional groups in Organic Chemistry.
Functional group analysis
Organic chemistry has a series of reactions that allow us to characterize almost all the functional groups present in molecules.
In some cases, several functional groups may give the same reaction. Therefore, it will be necessary to apply some other characteristic reaction to be sure of their nature.
In fact, some polyfunctional compounds can give reactions different from that of the various functions separately.
Therefore, the reactions listed below should not be taken as infallible and/or exclusive methods for the identification of functional groups.
For example, the test with iodine for the formation of the charge transfer complex is given for practically all functional groups, if it is indicated for alkenes it is because we assume that (after performing the solubility tests) it is a hydrocarbon and we want to know if it is saturated or unsaturated.
Although the characteristics of the IR spectrum are indicated, it is not a requirement without which the function cannot be identified, it would only accelerate or confirm it.
Identification of an unknown substance
As a summary of the qualitative organic analysis of recognition of an unknown substance we will follow this guideline:
- Physical examination of the product (organoleptic properties).
- Determination of physical constants.
- Qualitative elemental analysis.
- Study of the IR spectrum.
- Classification by solubility.
- Characteristic reactions of functional groups.
- Table study of the possible product.
- Preparation of solid derivatives.
- Product identification.
It is recommended to fill in the following sheet for each of the unknown products.
FUNCTIONAL ANALYSIS SAMPLE No. : ____
1.- ORGANOLEPTIC PROPERTIES AND PHYSICAL PROPERTIES:
a) State:_______________ Melting point:_____________
b) Color:________________ Boiling point:__________
c) Smell:_________________
2.- ELEMENTARY ANALYSIS: Contains:_______________
3.- INFRARED SPECTRUM:
Absorption (cm-1) Assignment
_________________ ___________________
_________________ ___________________
4.- SOLUBILITY:
Soluble in:___________________________________________________
Insoluble In:__________________________________________________
Group:_________________
5.- ORGANIC FUNCTIONAL ANALYSIS: Indicate reagent, result and deductions.
a)____________________________________________________________________
b)____________________________________________________________________
6.- POSSIBLE PRODUCTS (Indicate the reason):
______________________________________________________________________
7.- FORMATION OF DERIVATIVES:
a)____________________________________________________________________
b)____________________________________________________________________
8.- PRODUCT IDENTIFICATION:
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145