Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the solutions to the problems.
Haloalkanes – Alkyl Halides – problem list
Problem 1:
Classify the type of halide (primary, secondary, tertiary, allylic or benzyl) from the following list and draw the carbocation(s) obtained from each.
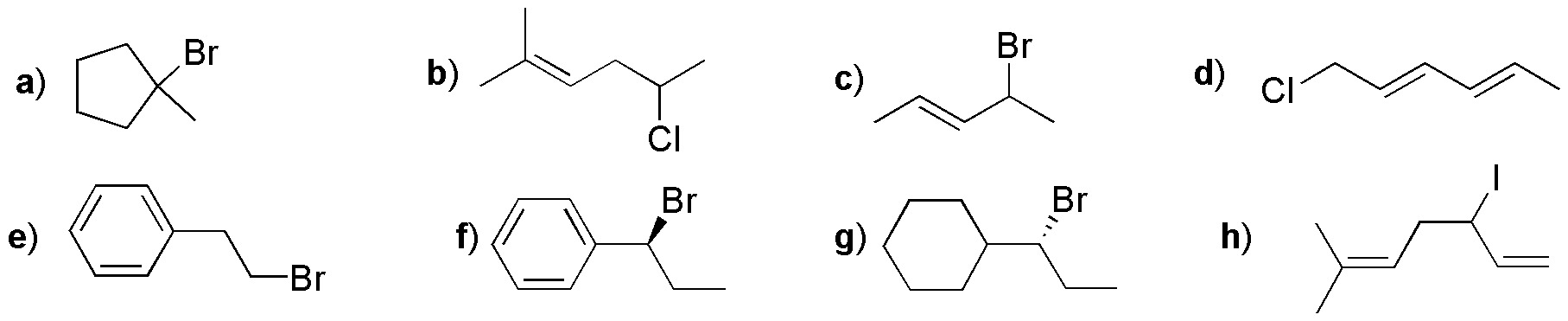
Problem 2:
Classify the type of halide (primary, secondary, tertiary, allylic or benzyl) from the following list and draw the carbocation(s) obtained from each of them.
Problem 3:
(a) draw the structures of the following compounds:
- 1-chlorobutane (I)
- 2-chloro-2-methyl-propane (II)
- 3-chloroprop-1-ene (III)
b) Order these compounds from highest to lowest reactivity for the SN1 reaction.
Problem 4:
(a) Draw the structures of the following compounds:
- 2-chloro-2-methylpropane (I)
- 1-chloropropane (II)
- 2-chloropropane (III)
- chloromethane (IV)
(b) Order these compounds from highest to lowest reactivity for reaction SN2.
Problem 5:
Describe the mechanism of the reaction of formation of 2-methoxy-2-methylpropane from 2-chloro-2-methylpropane when the latter is heated in methanol.
Problem 6:
Draw the structure of the reaction product of methyl bromide with the following substances:
- Hydrogen sulfide
- Bisulfide ion
- HO–
- Ethylamine
- Sodium cyanide
- Ethanol
Problem 7:
What nucleophiles will be necessary to obtain the following compounds from CH3-CH2-CH2-CH2-CH2-Br ?
- CH3-CH2-CH2-CH2-CH2-NHCH3
- CH3-CH2-CH2-CH2-CH2-COCH3
- CH3-CH2-CH2-CH2-CH2-OC6H5
- CH3-CH2-CH2-CH2-CH2-SCH2CH3
- CH3-CH2-CH2-CH2-CH2-OH
Problem 8:
2-bromo-2-methylpropane when dissolved in acetic acid is transformed into 2-methylbutan-2-yl acetate.
a) Define the type of reaction.
b) Justify why if sodium acetate is added to the reaction medium no appreciable variation in the rate of reaction is observed.
Problem 9:
When tert-butyl bromide is treated with an aqueous solution of silver nitrate, the formation of a precipitate is observed. Propose a reaction mechanism compatible with this fact.
Problem 10:
Indicate which molecule of the following pairs reacts most rapidly with sodium azide in acetone:
- 2-bromopropane vs. 1-bromopentane
- 2-bromopropane vs. 2-chloropropane
- 1-bromopentane vs. 1-bromocyclohexane
Problem 11:
1-bromopropane reacts with sodium methoxide in methanol to give methyl propyl ether in 90% yield, whereas 1-bromo-2-methylpropane under the same conditions gives isobutyl methyl ether only in 40% yield. Justify the result.
Problem 12:
The substitution reaction between 1-iodopropane and cyanide ion is of type SN2. Draw the reaction profile and explain what happens when the concentration of cyanide ion is halved in the reaction medium.
Problem 13:
Of the following pairs of reactants, indicate which would be a better nucleophile:
a) Cl– / I–; b) CH3S– / CH3O–; c) H2O / HO–; d) CH3O– / F–
Problem 14:
1-iodo-2-methylbutane reacts independently with the following reagents:
I) NaOH / MeOH
II) NaOH / DMSO
III) NaSH / MeOH
IV) NaSH / DMSO
V) H2O
a) Draw the product obtained in each case.
b) Deduce which will be the fastest reaction in the series.
Problem 15:
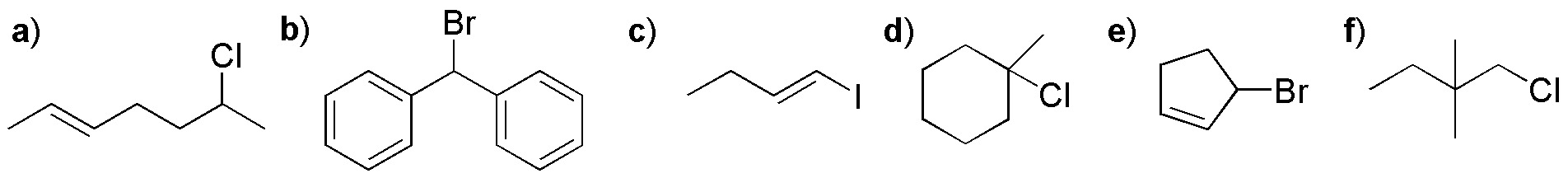
Which type of reaction (SN1 or SN2) is most likely to occur in the following transformations?
Problem 16:
Complete the following scheme:
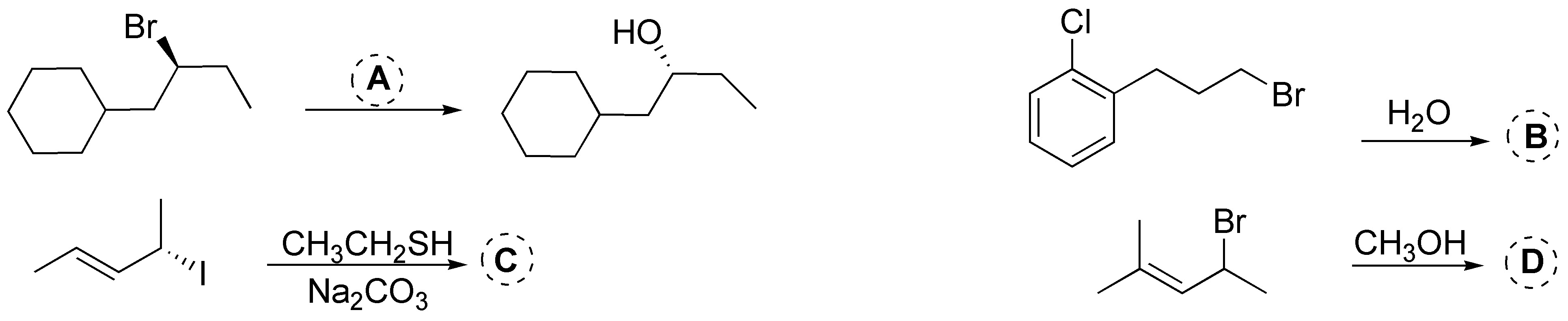
Problem 17:
Rank the following halides in increasing order of reactivity for an SN2 type reaction and against the same nucleophile.

Problem 18:
Rank the following substrates in decreasing order of solvolysis rate.
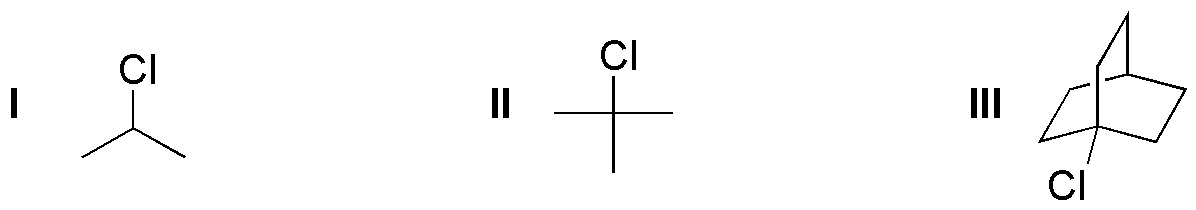
Problem 19:
Propose a mechanism that justifies the following transformation:
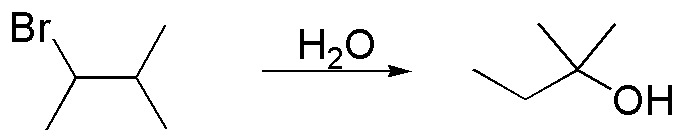
Problem 20:
When 4-chloro-butanol is treated with a solution of NaOH, THF is obtained. Justify the result.
![]()
Problem 21:
The following molecules can give under the appropriate conditions elimination reactions.
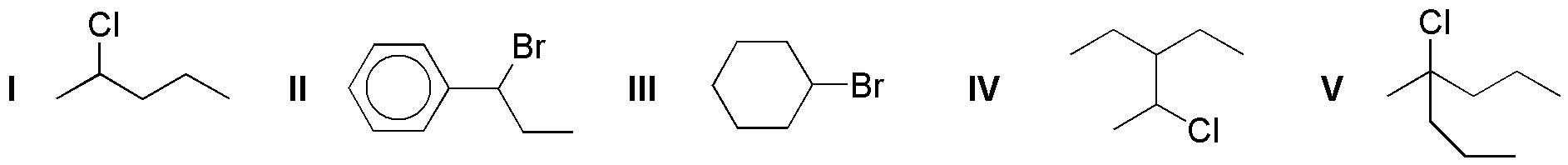
a) Mark with a dashed circle the positions of the α-hydrogens with respect to the leaving group.
b) Draw the structure of all the alkenes obtained in each case, indicating the one formed in the highest proportion for the usual conditions of the reaction.
Problem 22:
Complete the following reactions and derive the rate equation for each.

Problem 23:
Draw the structure of the compounds obtained under the appropriate conditions when the following compounds are treated independently with NaCN in acetone and methanol.
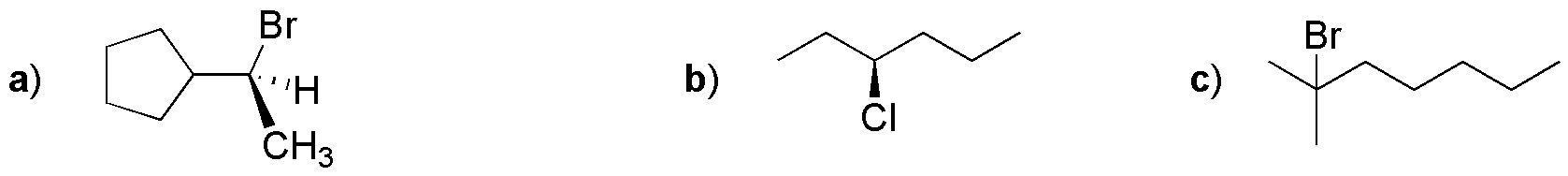
Problem 24:
The reaction of (S)-3-bromo-3-methylhexane with methanol leads to a mixture of products.
a) Indicate how the concentration of methanol affects the final result.
b) Properly represent the structures of the compounds obtained.
Problem 25:
Choose the appropriate reagent to perform the following transformations.
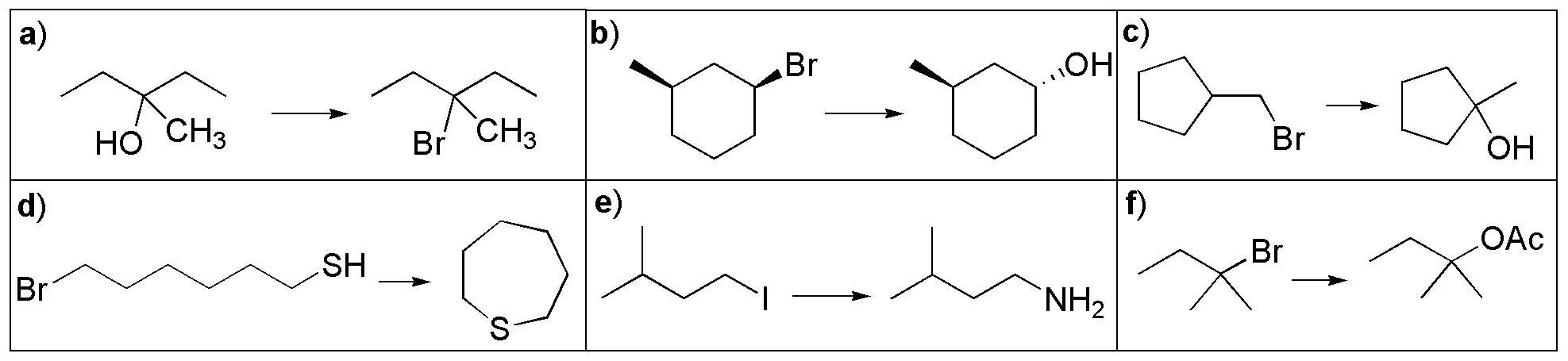
Problem 26:
Select the mechanism (SN1, SN2, E1, E2, NO React) under the following reaction conditions:
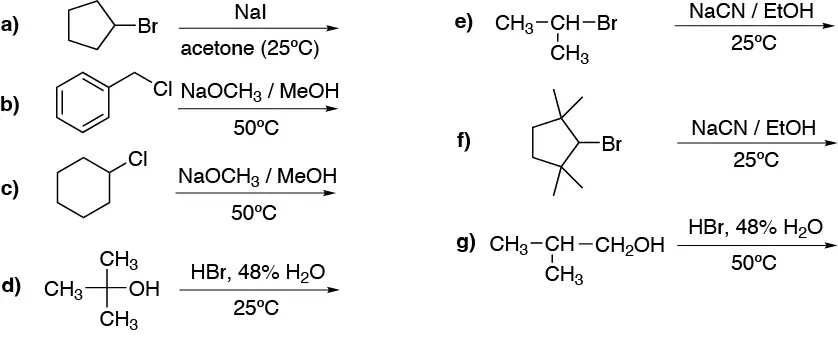
Problem 27:
(1R,2R)-1-bromo-2-ethylcyclohexane and (1S,2R)-1-bromo-2-ethylcyclohexane are treated independently with alcoholic potash in ethanol. Indicate in each case the result of the reaction.
Problem 28:
Which of these compounds generates a single alkene when treated with sodium methoxide?
- 2-bromo-2-methylpentane
- 3-bromo-3-ethylpentane
- 3-bromo-2-methylpentane
- 2-bromo-4-methylpentane
- 2-bromo-3-ethylpentane
Problem 29:
Complete these transformations (A-F) and explain in a reasoned way which will be the most likely mechanism in each case.
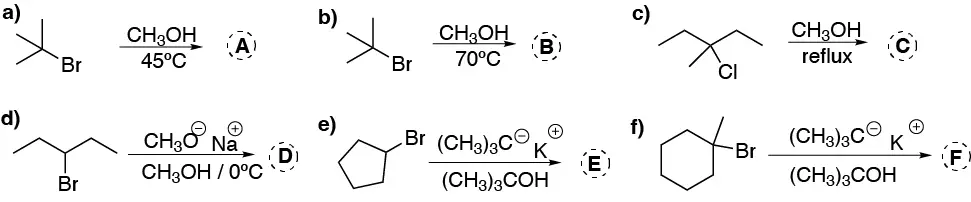
Problem 30:
Deduce which reactions will be faster from the following pairs:
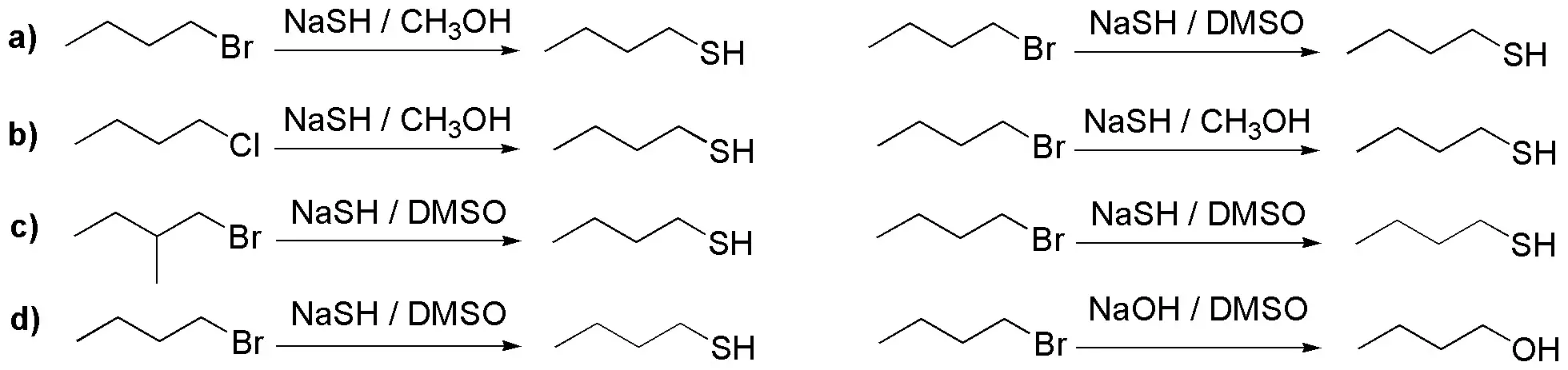
Problem 31:
When (R)-3-bromo-2,3-dimethylpentane is dissolved in an equimolecular mixture of water and methanol, four products different from the starting one are obtained after a time. Justify this result.
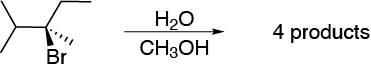
Problem 32:
Choose the most favorable conditions for the following transformations to occur:
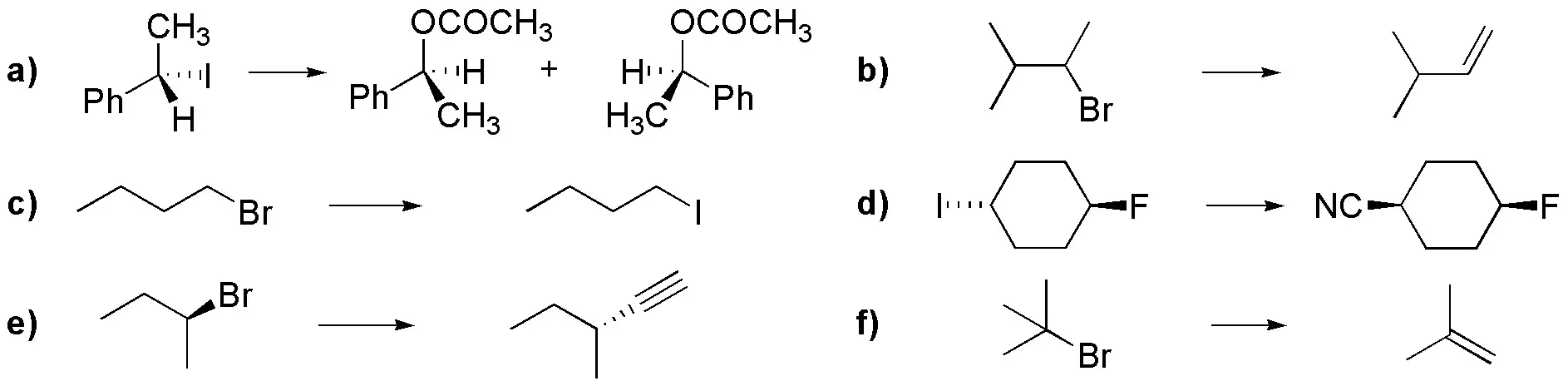
Problem 33:
Applying Zaitsev’s rule deduce which is the most stable alkene.
- 1-methylcyclohexene
- 3-methylcyclohexene
- 4-methylcyclohexene
Problem 34:
Draw the structure of the major compound obtained by treating the following molecules with sodium ethoxide in ethanol.
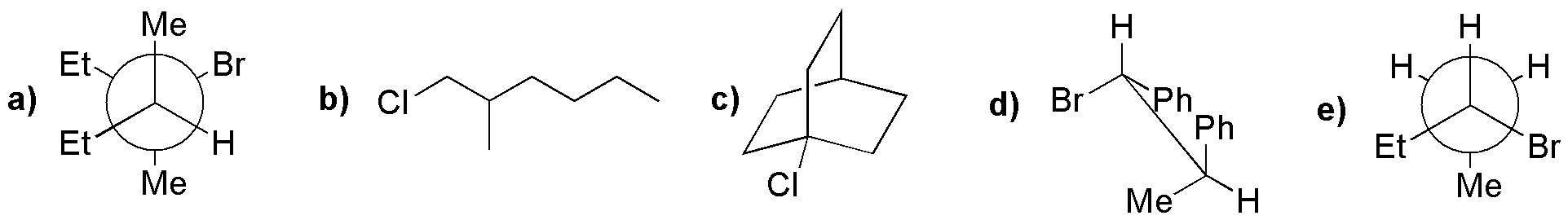
Problem 35:
Justify the higher reaction rate of I (cis) versus II (trans) in the E2 reaction.
![]()
Problem 36:
1-Iodo-2-methylpropane is treated with sodium metal to give 2,5-dimethylhexane and as by-products 2-methylpropane and 2-methylpropene. Justify the results.
Problem 37:
Complete the following scheme:
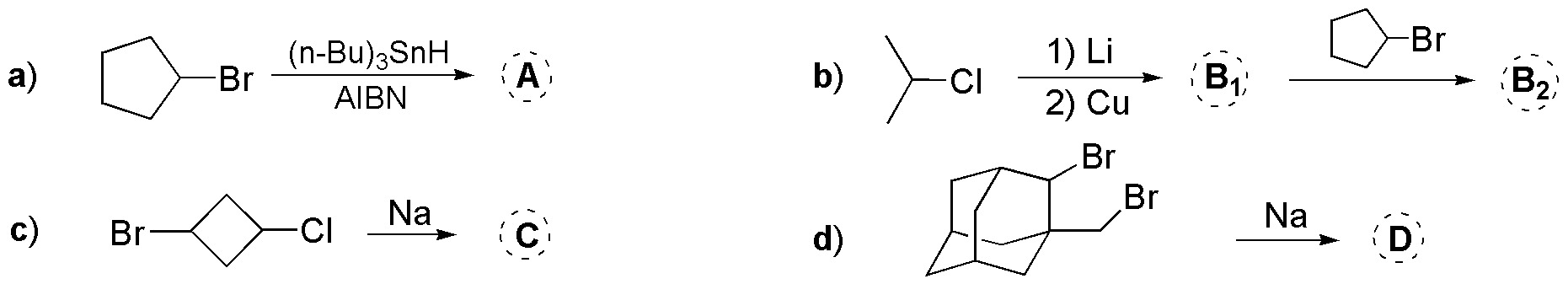
Problem 38:
Complete the following scheme:
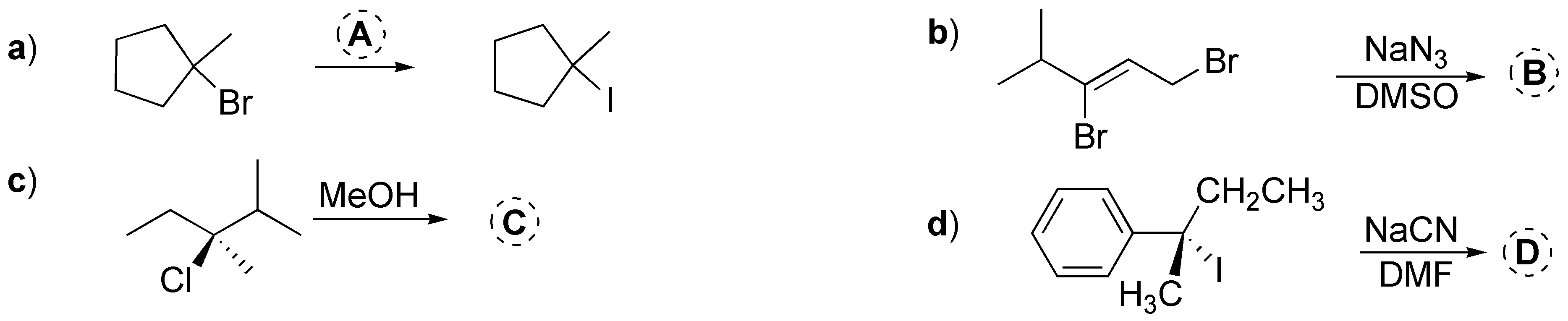
Problem 39:
Arrange the following halides in increasing order of reactivity in an SN2 reaction against the same nucleophile.

Problem 40:
Choose the nucleophiles and solvents you consider most suitable, from those listed below, to perform the following transformations:
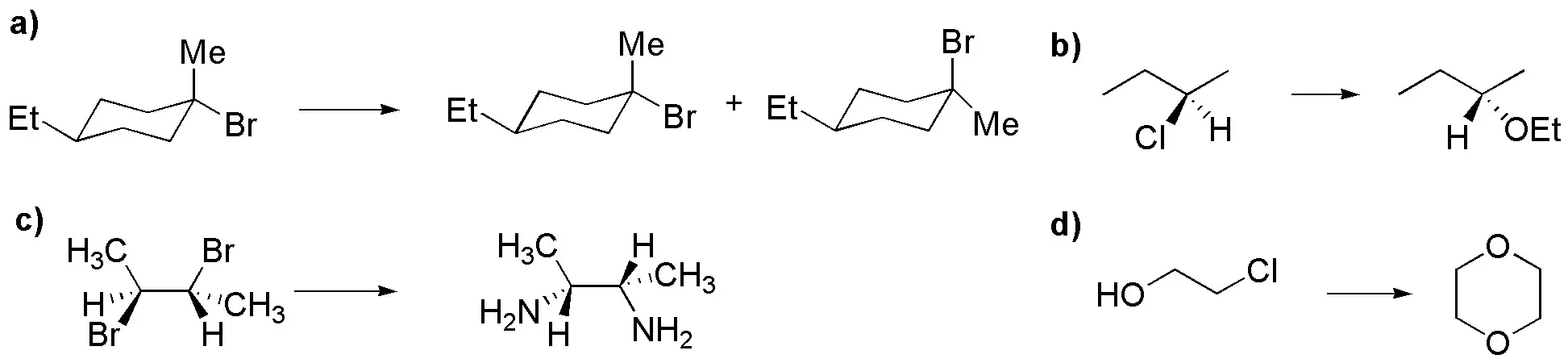
Problem 41:
Rank in increasing order of solvolysis rate the following substrates.

Problem 42:
Complete the following reactions and deduce the most likely type of reaction (E1, E2, SN1, SN2).
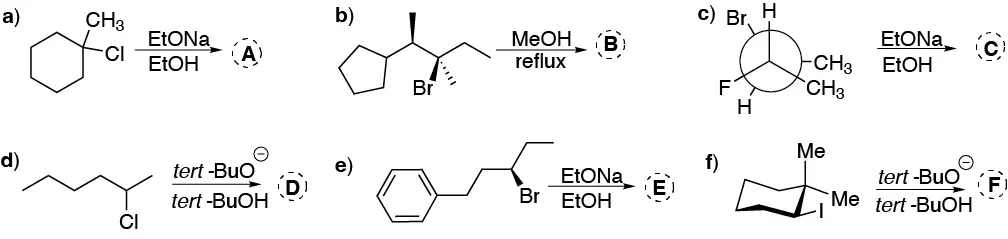
Problem 43:
Justify why 3-bromopentane exhibits a higher nucleophilic substitution reaction rate than 1-bromo-2,2-dimethylpentane, despite being a secondary halide.
Problem 44:
State what would be the major product of E2 elimination when the following products are treated with KOH in ethanol:

Problem 45:
When E2 elimination of the following products occurs:
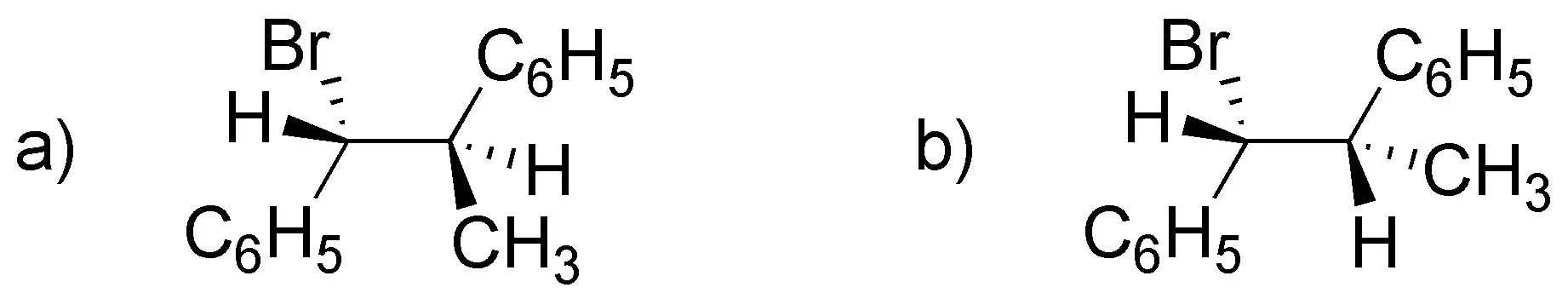
one of them performs it 50 times faster than the other. which of the two isomers is and why?
Problem 46:
What will be the result of treating the following haloalkanes with NaOEt/EtOH or with t-BuOK/t-BuOH?
- a) Chloromethane
- b) 1-Bromopentane
- c) 2-Bromopentane
- d) 1-chloro-1-methylcyclohexane
- e) 1-Bromoethylcyclopentane
- f) (2R,3R)-2-chloro-3-ethylhexane
- g) (2R,3S)-2-chloro-3-ethylhexane
- h) (2S,3R)-2-chloro-3-ethylhexane
Problem 47:
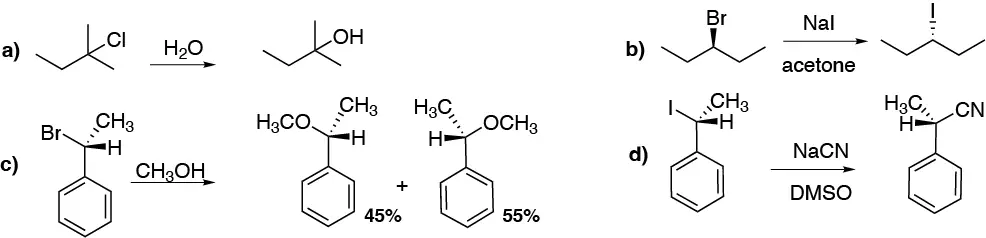
Explain why the following experimental results:
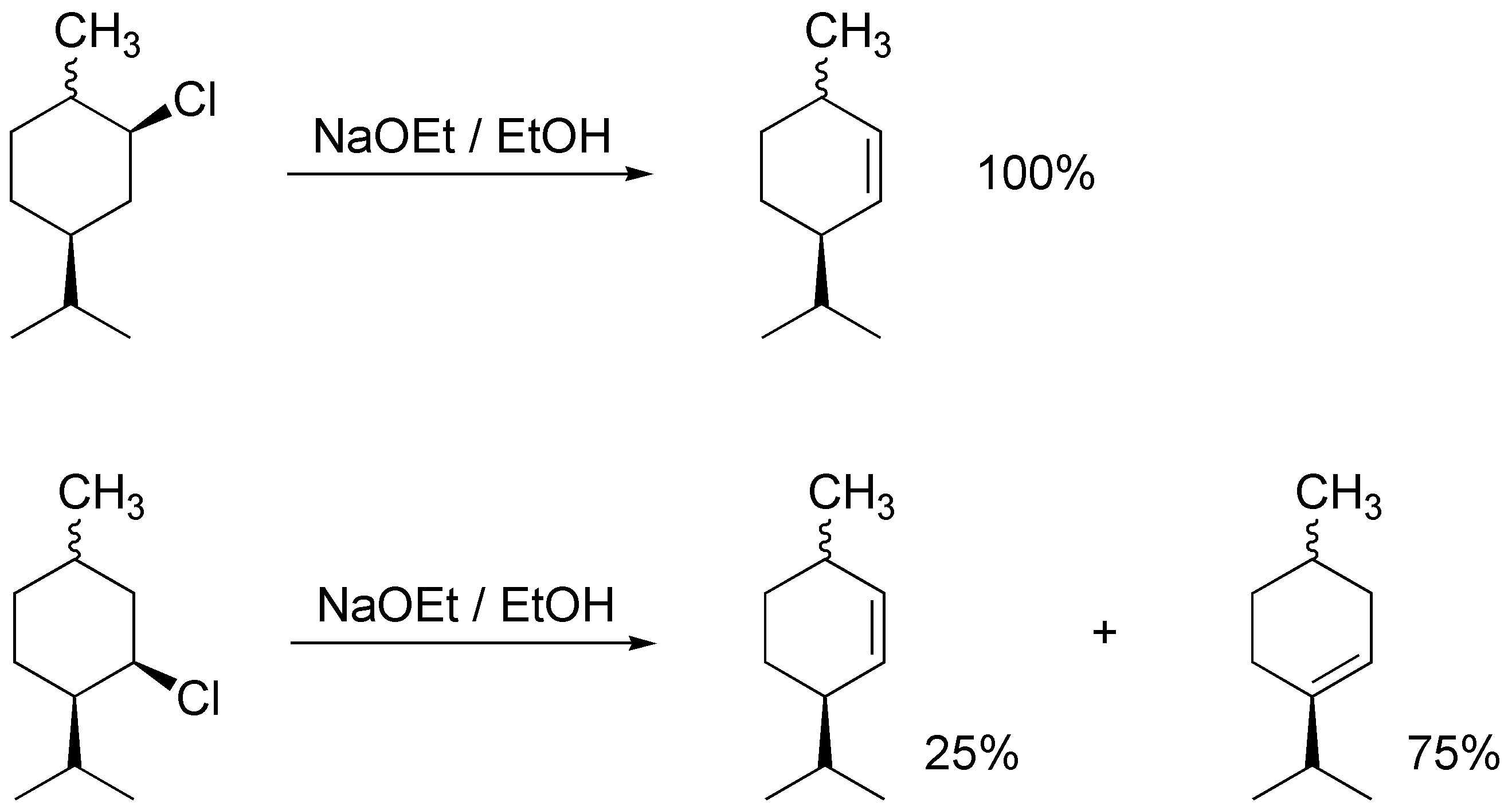
Problem 48:
Describe what are all the possible products of the acetolysis (CH3COOH) of 1-iodo-1-methylcyclohexane and 2-iodo-1-methylcyclohexane.
Problem 49:
Describe the result of treating (3S,4S)-3-bromo-4-methylhexane and (3S,4R)-3-bromo-4-methylhexane independently with:
(a) alcoholic potash; (b) potassium tert-butoxide; (c) sodium azide; (d) tri-n-butyltin hydride.
Problem 50:
Considering Zaitsev’s rule, select the most stable alkene.
- 1,2-dimethylcyclohexene
- 1,6-dimethylcyclohexene
- cis-3,4-dimethylcyclohexene
Problem 51:
Choose the starting product (I-X) and the most suitable reaction conditions for obtaining the following molecules (a-f) by elimination reactions.
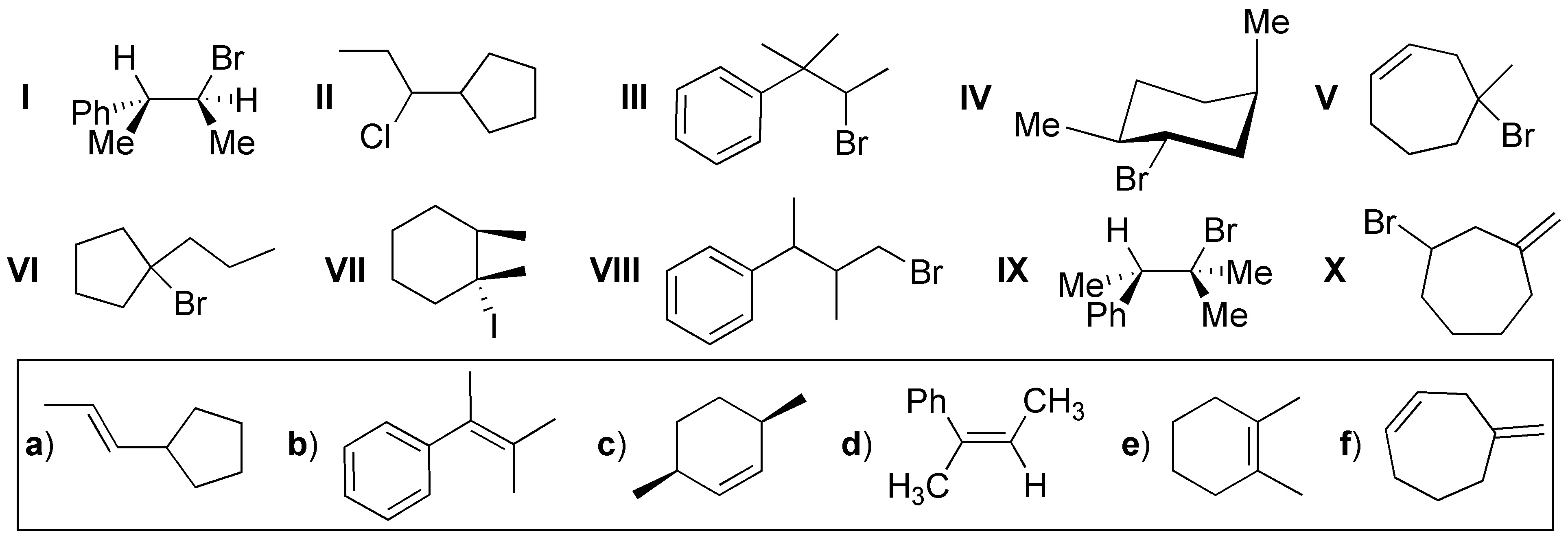
Problem 52:
Independently treat the relative cis- and trans-stereochemistry isomers of 1-chloro-2-methylcyclohexane with alcoholic potash. Analyze the outcome of these reactions employing the Newman projection for each case.
Problem 53:
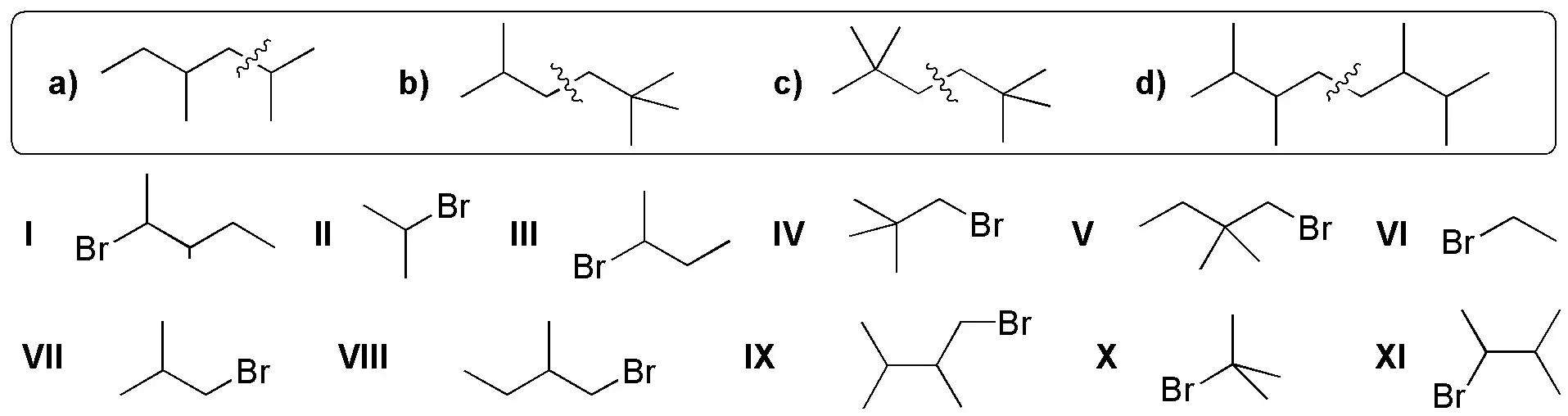
Draw the structure of the starting products that you consider most suitable for preparing the following hydrocarbons by Corey-House synthesis, such that the carbon skeleton marked with thick stroke is incorporated into the final product from a single product.
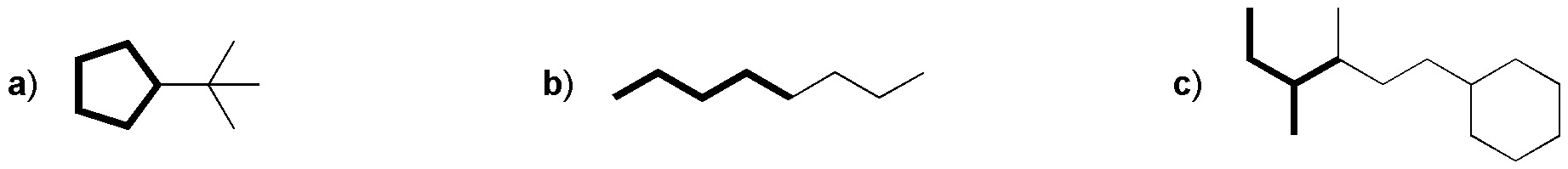
Problem 54:
What product is obtained from the following reactions?
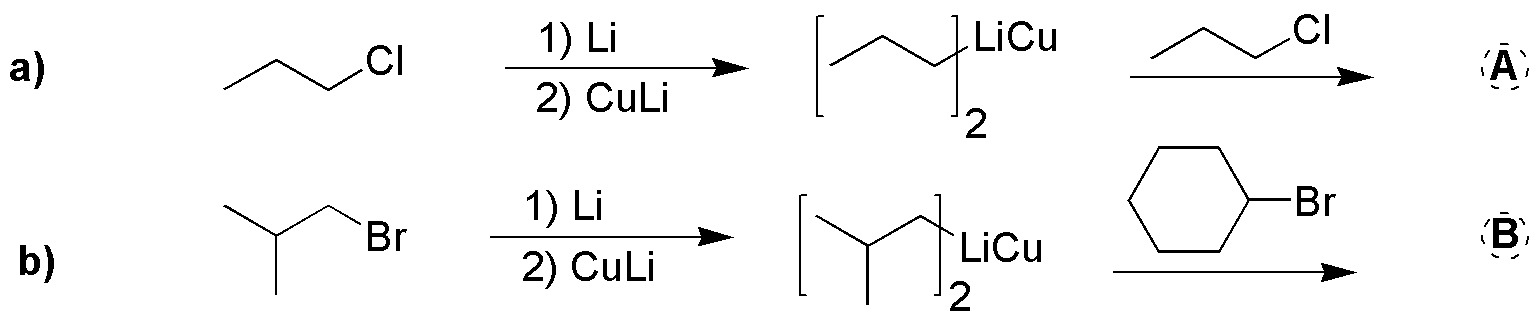
Problem 55:
Select the starting brominated derivatives (I-XI) needed to obtain, by Corey-House synthesis, the alkanes (a-d), where the C-C bond formation points are indicated.