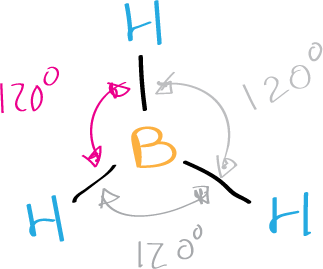Written by J.A Dobado | Last Updated on May 2, 2024
What is the Lewis structure of borane BH3?
Borane, also known as boron trihydride, is a chemical compound consisting of one boron atom and three hydrogen atoms. The Lewis structure of borane BH3 is a way of representing its molecular structure using symbols to show the bonding between the atoms and the electrons involved in the bond formation.
To draw the Lewis structure of borane, we first need to determine the total number of valence electrons in the molecule. The valence electrons are the electrons in the outermost shell of the atom that participate in chemical bonding. Boron has three valence electrons, and hydrogen has one valence electron each, so the total number of valence electrons in borane is:
3 (valence electrons of boron) + 3 x 1 (valence electrons of hydrogen) = 6
Next, we place the atoms in the structure, with the boron atom in the center and the three hydrogen atoms arranged around it. Each hydrogen atom is bonded to the boron atom with a single covalent bond, which means they share one pair of electrons.
After the bonding, we can see that boron still has only six electrons, which is not enough for its octet to be complete. To fulfill the octet rule, we can create a coordinate covalent bond between boron and one of the hydrogen atoms, where the lone pair of electrons on the hydrogen atom is used to form a bond with boron. This gives boron a total of eight electrons, completing its octet.
The final Lewis structure of borane BH3 looks like this:
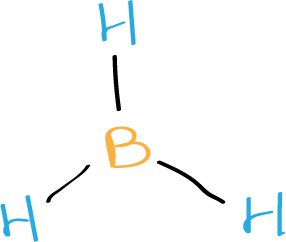
In this structure, each hydrogen atom is connected to the boron atom by a single covalent bond, and one of the hydrogen atoms is also connected to boron by a coordinate covalent bond.
The Lewis structure of borane BH3 shows that it is a trigonal planar molecule, with a bond angle of 120 degrees between the hydrogen atoms. It is important to note that borane BH3 is a highly reactive and unstable compound, and it readily reacts with other molecules to form more stable compounds.