Written by J.A Dobado | Last Updated on May 2, 2024
What is the Lewis structure of nitrogen trifluoride NF3?
The Lewis structure of nitrogen trifluoride (NF3) consists of three N-F bonds and a lone pair on the nitrogen atom, while each fluorine atom has three lone pairs.
Drawing the Lewis structure of NF3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial.
Once the Lewis structure is drawn, the shape of the NF3 molecule can be determined.
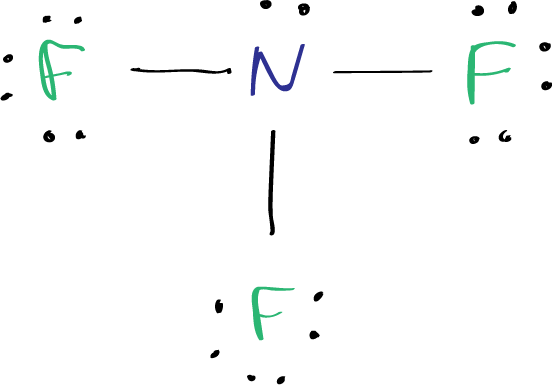
The Lewis structure of NF3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs.
In the Lewis structure of the NF3 molecule, a central nitrogen atom is surrounded by three fluorine atoms. Each nitrogen–fluorine bond is represented by a single line, and there is a lone pair of electrons on the nitrogen atom.
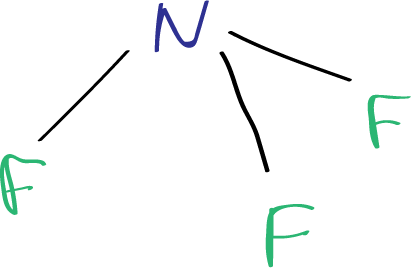
The three fluorine atoms are arranged in a trigonal pyramidal geometry (such as ammonia molecule), with each fluorine atom positioned at a 102.5-degree angle from the others.
Step by step drawing the Lewis structure of NF3
To draw the Lewis structure of NF3, a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions.
These steps include:
- determining the total number of electrons in the valence shells of the nitrogen and fluorine atoms
- identifying the total number of electron pairs as lone pairs and bonds
- selecting the central atom
- marking the lone pairs on the atoms
- marking any charges on the atoms
Finally, the stability of the structure needs to be checked, and any charges on the atoms minimized by converting lone pairs to bonds to obtain the best Lewis structure.
Total number of valence electrons
The total number of valence electrons in NF3 can be determined by considering the two elements present: fluorine and nitrogen.
Fluorine, a group VIIA element, has seven electrons in its valence shell, while nitrogen, a group VA element, has five electrons in its valence shell.
To calculate the total valence electrons, the number of electrons in the valence shell of each element is multiplied by the number of atoms of that element present in the molecule.
For NF3, there are three fluorine atoms and one nitrogen atom.
Thus, the valence electrons given by the three fluorine atoms would be 7 x 3 = 21, and the valence electrons given by the nitrogen atom would be 5 x 1 = 5.
Therefore, the total valence electrons in NF3 would be 21 + 5 = 26.
Total valence electrons pairs
To determine the total valence electron pairs in a molecule like NF3, one needs to consider the number of σ bonds, π bonds, and lone pairs present in the valence shells. This value is equal to the total number of valence electrons in the molecule divided by two. In the case of NF3, the total number of valence electrons is 26, resulting in a total of 13 valence electron pairs.

Center atom
When selecting the center atom in a molecule, it is important to consider both the ability to have a greater valence and to be the most electropositive element. Nitrogen, with a maximum valence of 5, has a higher valence than fluorine, which has a maximum valence of 1. Additionally, nitrogen has a lower electronegativity value (2.1) than fluorine (4.0).
Therefore, nitrogen is the most suitable element to be the center atom in the NF3 molecule.
How many lone pairs of electrons are on nitrogen in nf3?
In NF3 (nitrogen trifluoride), the nitrogen atom has one lone pair of electrons. This is because nitrogen has five valence electrons, and it forms three covalent bonds with fluorine atoms, leaving one pair of electrons unshared or in a lone pair.
Lone pairs on atoms
After determining the center atom and sketching the NF3 molecule, the next step is to mark the lone pairs on the atoms, considering the total of thirteen electron pairs.
Since there are already three N-F bonds in the molecule, there are ten (13–3) electron pairs left to be marked on the atoms. Typically, the remaining electron pairs are marked on the outside atoms first, which in this case are the fluorine atoms. Therefore, three lone pairs will be marked on each fluorine atom, totaling 9 electron pairs.
Finally, the remaining 1 lone pair can be marked on the nitrogen atom.
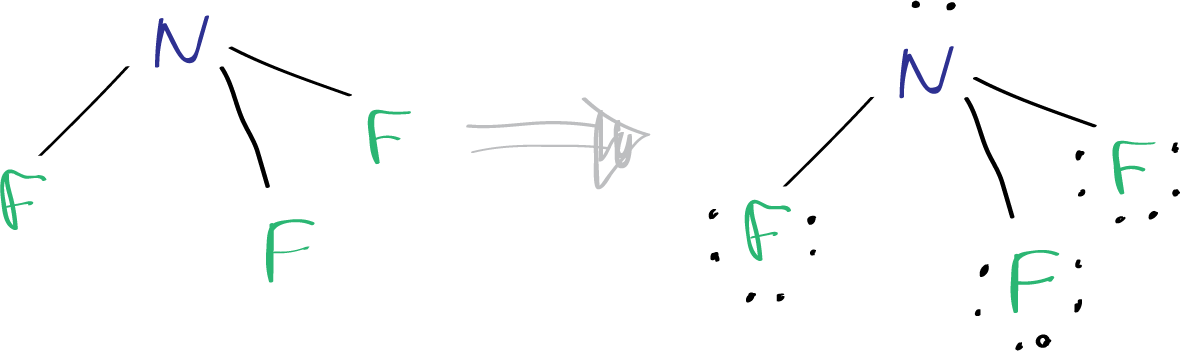
Mark charges on atoms and reducing charges
As there are no charges present on either the nitrogen or fluorine atoms in the NF3 molecule, we don’t need to consider reducing any charges on the atoms to achieve the most stable structure. So, we have successfully obtained the Lewis structure of NF3.