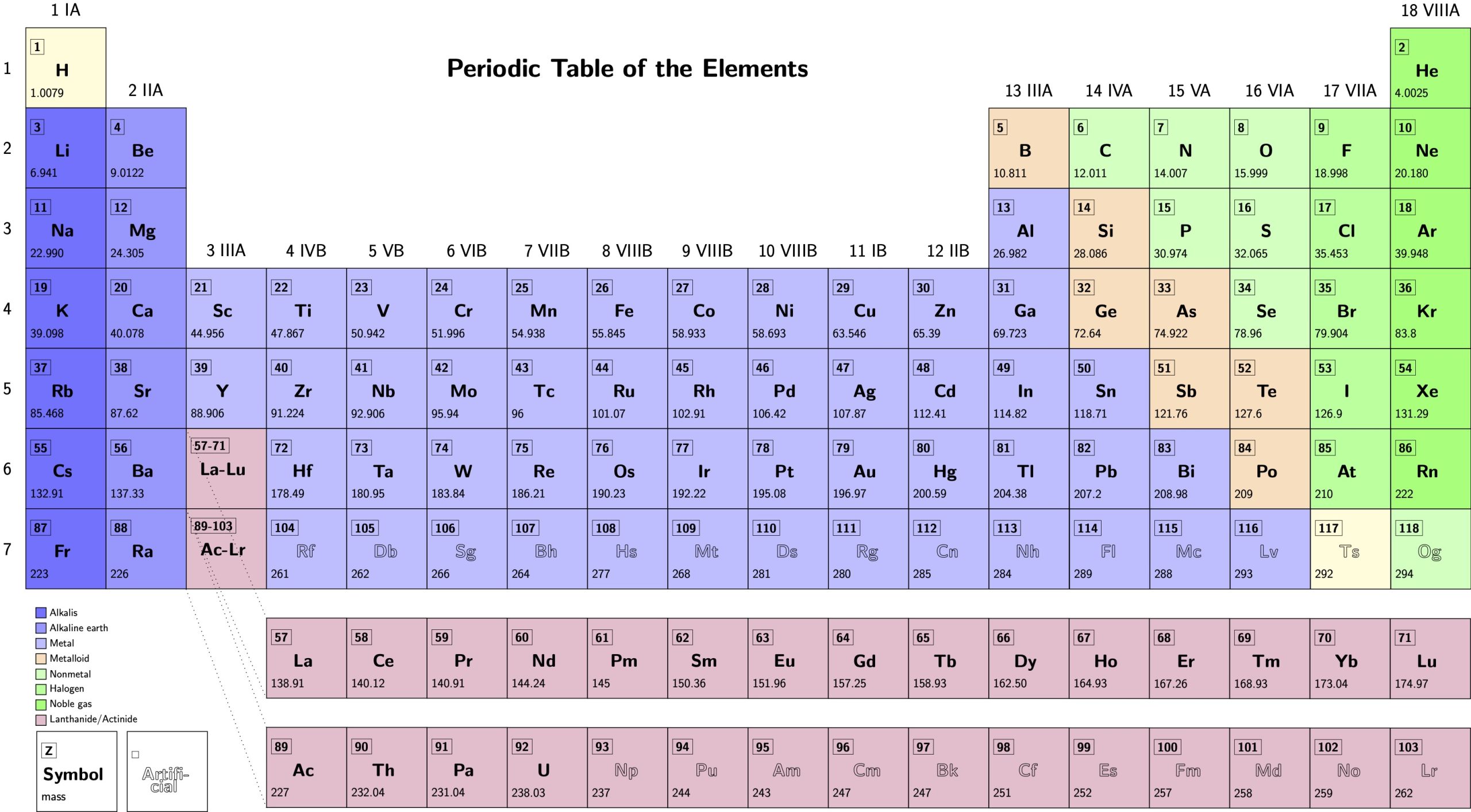
The current periodic table of the elements is as follows:
Origin of the Periodic Table
Is called periodic table the representation of the chemical elements in an orderly manner, in groups and periods proposed by the Chemist Dmitri Mendeleiev (1834-1907). In 1869, Mendeleev ordered the elements known to date in the form of a table where they were repeated properties in groups, when you explained to your students with letters in which were listed the atomic weight, and various chemical properties.
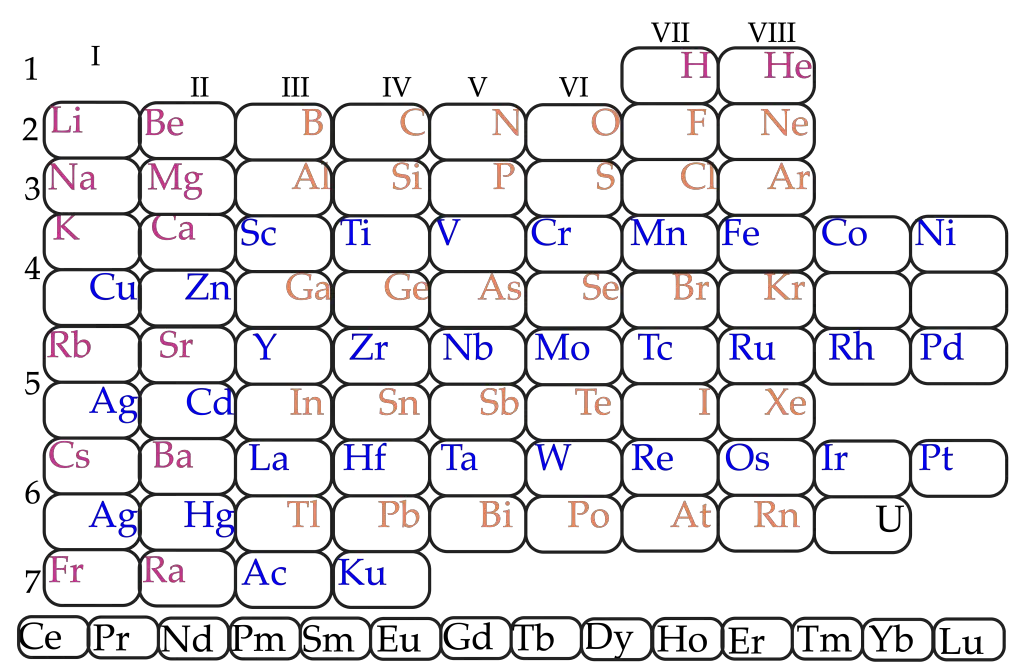
When ordering so increasing the atomic weight were the elements of a same group showed similar chemical properties.
Elements ordered by atomic number
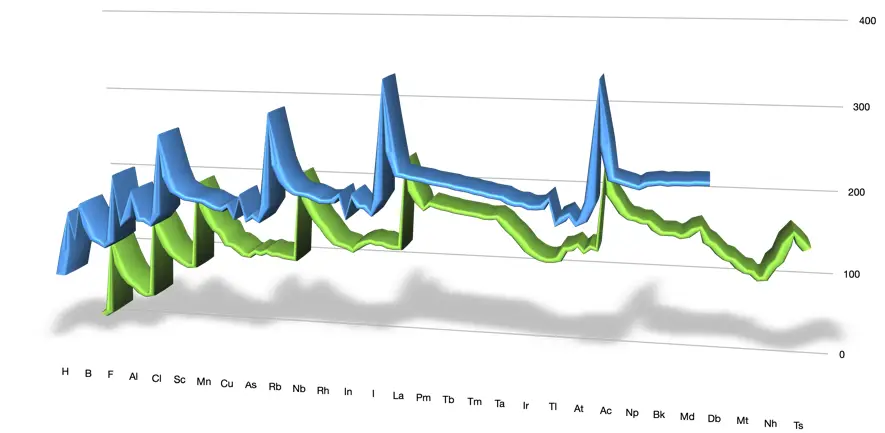
How does the atomic radius vary?
The following table shows the values of the van der Waals and covalent radii for all the elements of the periodic table
The following figure illustrates how these values vary periodically as we increase the atomic number of all the elements.
Physicochemical properties of the elements
Atomic mass and abundance of different isotopes
The atomic masses of the different isotopes are listed below, together with their relative abundance.
Video about The Periodic Table
FAQ
What is the periodic table and examples?
The periodic table of the elements is a table in which all the chemical elements known to man are arranged in order according to their atomic number (number of protons), electron configuration and specific chemical properties.
How many elements are there in the periodic table 2022?
Currently, the periodic table is composed of 118 elements distributed in 7 horizontal rows called periods and 18 vertical columns, known as groups.
References and notes
- The data have been taken from CRC Handbook of Chemistry and Physics 97th Edition.