Written by J.A Dobado | Last Updated on April 22, 2024
What is Lab equipment?
The work in the laboratory requires the use of different tools that enable an efficient execution and basic techniques. There are in the market a great variety of designs and materials adapted to each type of task, which can be classified such material in very different ways. Below, we describe a number of implements (glassware and equipment), which are typically used in an Organic Chemistry lab.
Many of these items correspond to the so-called glassware[1]. This name comes from the fact that the basic parts are made of glass and in these the chemical processes take place, while the remaining parts other than glass, in most cases are auxiliary. However, in recent years there have appeared new polymeric materials that are replacing the glass for certain tasks, so it is also appropriate to describe these new materials.
General-purpose glassware
The labware is very diverse, both in its design as in its composition, because it adapts to the different needs, such as storage of products, make volumes and weights measures, performing a reaction, purification of substances, etc.
A fundamental part of thelabware is glass because it is resistant to most reagents, it is transparent, so it allows the observation of the processes and on the other hand, it is an easy material to work with and adapt to any form.
Many models, types and variants of tools are available on the market, making it unfeasible to describe, and classify them exhaustively. Many of these items are named for the chemists who designed them.
Assembly of lab equipment
To perform many of the basic laboratory operations, is required to perform a series of set-ups between various components. This coupling is performed through ground-glass joints or combination of threaded adjustments. This allows a hermetic connection between the different parts, preventing the escape of vapors or liquids.
Ground-glass joints
Ground glass is a technique that gives it a matte finish using a surface treatment with acid or sandblasting one side of the glass.
The ground-glass joints, called “conically tapered joints”, are the most widespread connections to assemble glassware quickly and easily, so that the different parts are sealed without liquid leaking or vapour escaping when heated.
For example, you can join a round-bottom flask with a reflux condenser. In addition, these joints are also used for glass stoppers.
One of the glass elements connects an inner surface (or female) of ground glass and the other ground glass surface faces outward (or male), both with the same taper to facilitate the adjustment. The female joint presents a flange (as discussed below) to facilitate gripping.
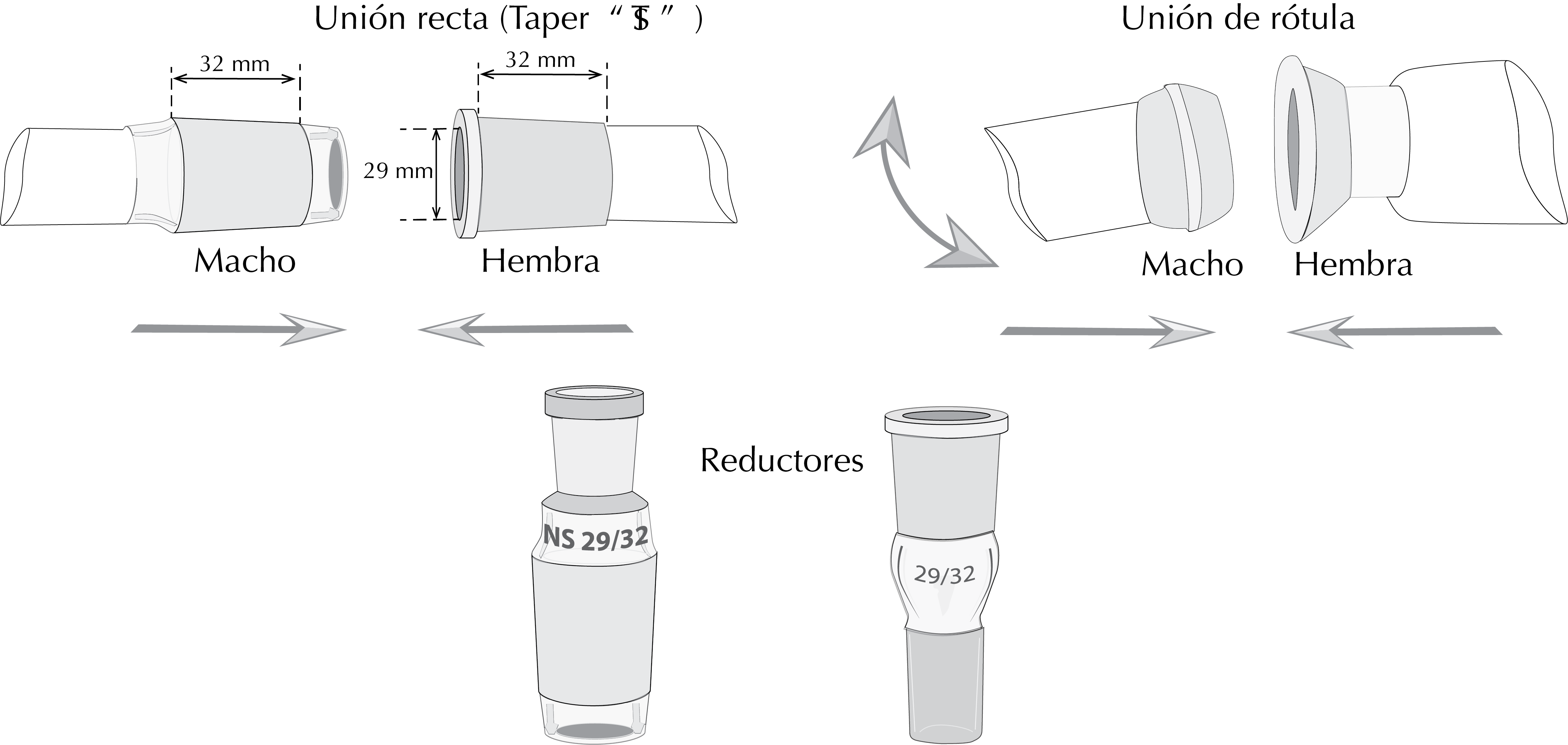
Standard Taper
The conical ground joints typically have a taper[2] referred to as 1:10 and are marked by a symbol consisting of a capital letter T, superimposed on a capital S, which stands for Standard Taper. This symbol is followed by a number, a slash, and another number. The first number represents the outside diameter in millimeters at the base (widest part) of the inner joint. The second number represents the ground glass length of the joint in millimeters.
The most common joints in the United States are 14/20 and 24/40. However in Europe it follows the European Standard ISO binding sizes 10/19, 14/23, 19/26, 24/29, and 29/32. US and European standards (ISO) differ only in length but not in the taper, and can be used in combination.
The caps of bottles of chemicals, flasks and funnels, and so on often do not use these standards. In Spain, the most usual are the joints 29/32 and 14/23. There are adapters that allow the assembly between components with different tapers both for expansion and reduction.
Hooks
Some ground-glass pieces presented two glass hooks 180º appart just above the grind that can be used to connect the joint with small metal springs. Although today they are in disuse, they serve to couple the parts and maintain the joint fastened. By ensuring joints with these springs, overpressure that may occur is prevented, and in these cases the joint opens and releases the pressure. However, caution must be taken in closing the joints because sometimes it is safer for overpressure to be released, resulting in a spill, because a firmly attached joint could cause to the glassware to explode. For example, HCl has a vapor pressure at room temperature, high enough to blow up the standard glass.
Lubrication
Silicone is often used to lubricate the stopcocks and ground-glass joints, preventing them from becoming blocked.
Although using lubricants keep joins from leaking, and subsequently, they can then separate the two parts more easily, however, have the disadvantage that it may contaminate the reaction products[3].
Threaded ground-glass joint
There are other interchangeable conical ground joints that improve the safety assemblies and provide sealing. There is no need for other elements to ensure the clorure (clip), as this kind of joints improves rigidity of the assembly.
Ball and socket joints
These parts end in male and female hemispherical ground surfaces that fit together. These joints are marked with a code consisting of two numbers separated by a slash.
The first number represents the outer ball diameter in milimiters at its base or inner diamerer in millimiters at the outermost part of the cavity; in both cases these are the maximum diameters of the joints.
The second number represents the inner diameter of the hole in the center of the ball or the cavity, which leads to the inner diameter of the tube that is attached to the joint. To keep both pieces together, a clamp allows some movement between the parts assembled by this system.
Other ground-glass joints
The grinding technique is also used for the manufacture of glass desiccators and weighing bottles. The glass desiccators have a ground edge of the mouth and a lid with ground glass that makes a perfect perfect lace clorure and vacuum of the system. The weighing bottle, often used to store or weigh solids, is a cylindrical glass containers constructed with a glass lids with ground glass.
| DANGER! “One of the precautions to consider with glassware manipulation is not exert unnecessary pressure on when assembling or disassembling the material. (ground-glass joints, rotary evaporators and flasks, thermometers embedded with rubber stoppers). Glass get stuck due to heating. In these cases, a gentle rotary motion is used to dislodge the material.“ |
Ground-glass joint flasks
Flasks, vessels designed to perform mixing or making reactions, are manufactured with borosilicate glass and therefore are resistant not only to heat but also to sudden temperature changes (heat-shock resistant).
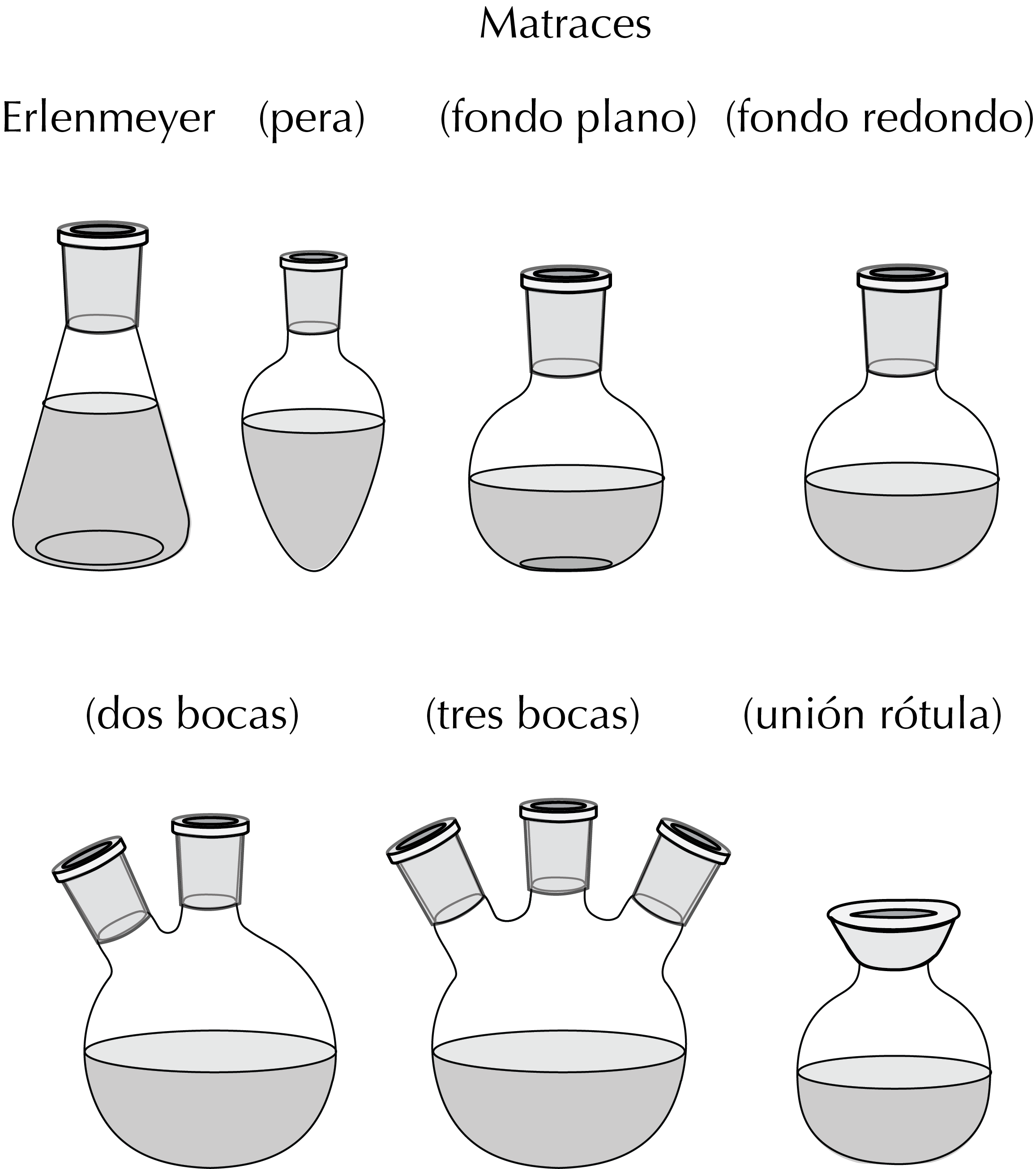
They may have different shapes such as a flat or round bottom. Following is a list of the most common ones:
- Ground-joint Erlenmeyer flask: conical in shape with a wider taper at the bottom than at the mouth.
- Round-bottom flask: can be completely spherical in shape or have a flat bottom.
- Pear-shaped flask: used in distillations or with a rotary evaporator.
- Volumetric flask: used to prepare solutions.
- Schlenk flask: used in air-sensitive chemistry and often connected to Schlenk lines.
All can vary from a few milliliters to several liters, and although the most common round-bottom flasks have one neck, some flasks have twin- or triple-necks for use in more comples assemblies.
Glassware without ground-glass joints
In this section, we describe some of the material frequently used in the lab, including glassware without ground joints.

Petri dish
It is a box formed by two circular parts that fit together that close but not tightly. Primarily used in microbiology for cell cultures. In Organic Chemistry it is used because of its low price to store solids (it is also manufactured in plastic).
Porcelain capsules
They are hemispherical with a peak in the mouth, to facilitate pouring, and are very useful to heat, dry, or to carbonize substances at high temperatures.
Crystallizing dish
They are cylindrical vessels with a wide base and a low height that are often used to prepare water, oil, or sand baths, or serve as a container for different materials. The name comes from their original use to obtain a solid by crystallization from solution.
Conical funnel
It is made of glass or plastic and is used to transfer solids or liquids. Funnels for solids have the widest tube to facilitate passage of the solid.
Erlenmeyer flask
The Erlenmeyer flask is of the most common flask in the laboratory and there are also variants with ground mouth.
Beaker
They are cylindrical containers with a flat bottom and a spout or lip, at the mouth to facilitate the transfer of liquids. They are very common among flasks without grinding. Beakers can hold from a few milliliters to several liters.
Washing bottle
These plastic or glass bottles have a perforated plug with a thin tube forming an angle, which is used to contain solvents.
Mortar and pestle
Often used for mixing or shearing solids may be manufactured with glass, ceramic, or agate. Soids are ground by making gentle circular movements with the pestle resting on the mortar, without banging.
Touch plate
Manufactured generally in porcelain, usually white, these have a series of cavities or wells used to perform tests and assays on a small scale.
Pasteur pipette
This is a transparent glass tube open at both ends but with a pronounced narrowing at one. The upper end is coupled with a rubber or latex bulb while the lower end of the tube tapers to a a capillary. It is used to transfer small amounts of liquids. It is manufactured in plastic in one piece, which it is not necessary to couple to a rubber bulb.
Plugs
There are a wide variety of plugs[4] commonly used in the laboratory, made of glass, rubber, Teflon, or other polymers such as PE, which today have replaced the cork. Septa caps (septum in the singular) are widely used for easily and tightly closing glass, ground and without ground-glass joints, such as Erlenmeyer flasks round bottom flasks, bottles or jars.
The cap is adjusted and closed to the flask mouth by folding over the outside of the septum. The main advantage is that it can be punctured with a needle or cannula, to introduce a syringe or reactive gas and to work in an inert atmosphere.
For flasks with a ground mouth, if a snug fit is required to prevent liquids or vapors from scaping, ground-glass or Teflon stoppers are used.
Test tube
These are cylindrical glass tubes, with one open end (top) and the other hemispherical (bottom). They are manufactured to be heat resistant or not, depending on the type of glass, with many applications in a laboratory. Test tube holders, designed for storing or handling several test tubes at a time, can be wood, metal, or plastic.
Watch glass
This is circular in shape and slightly convex, and it is used to weigh or dry solids.
Stopcoks
Many laboratory utensils have built-in stopcocks to open or close the passage of liquids or gases. They come with a glass body with interchangeable male and female ground-glass or Teflon joints.Ground glass joints require a good lubrication with silicone grease to prevent the stopcock locks.
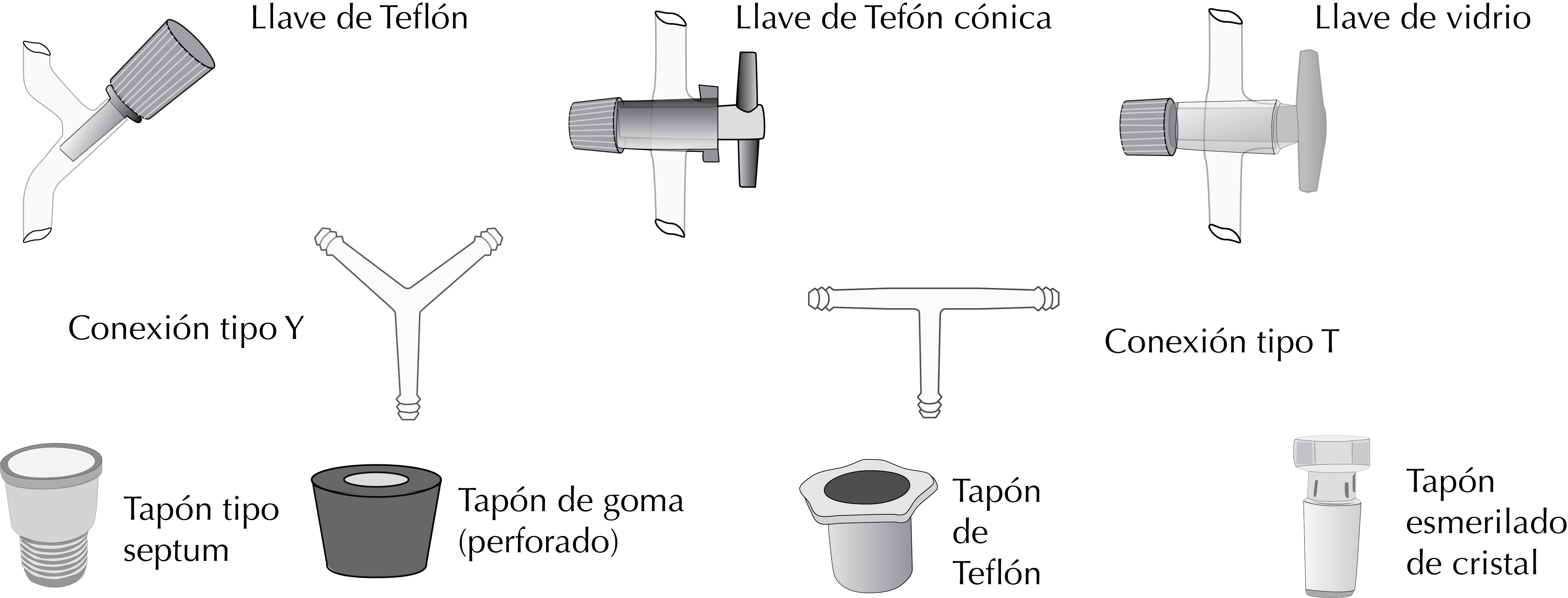
Stopcocks are also used for connecting tubes, for example, rubber, Teflon or silicone; for circulating fluids; to block the passage of fluids, or to control the air input or output in vacuum systems. These valves that connect tubing have a glass segment to the other side of the key of the stopcock with a widening conical end, so that the tube fits snugly and does not slip once connected. Some stopcocks are made entirely of plastic.
Connectors
Flexible tubing is assembled with glass or plastic connectors which may be straight, T-shaped, or Y-shaped.
Flexible tubing
In laboratories, there are different types of flexible tubing as well as pinchcock tubing clamps for liquid or gas circulation. Tubing may be of latex, silicone, neoprene, PVC, Teflon® etc, depending on the fluid being used and the temperature. They can be used for both gas and liquid flow.

In this type of tubes, it is sometimes necessary to regulate the flow rate. it is used a special tool (Hoffman and Mohr clamps) that allow you to reduce partially or even completely the gas or liquid flow at any point in a circuit consisting of these flexible tubes.
Other material
Listed below are a number of utensils of frequent use in the laboratory that have not been collected in the previous sections.
Glass rod
These are solid glass cylindrical, which is used to stir solutions, dissolve, handling solids in the funnel, etc.
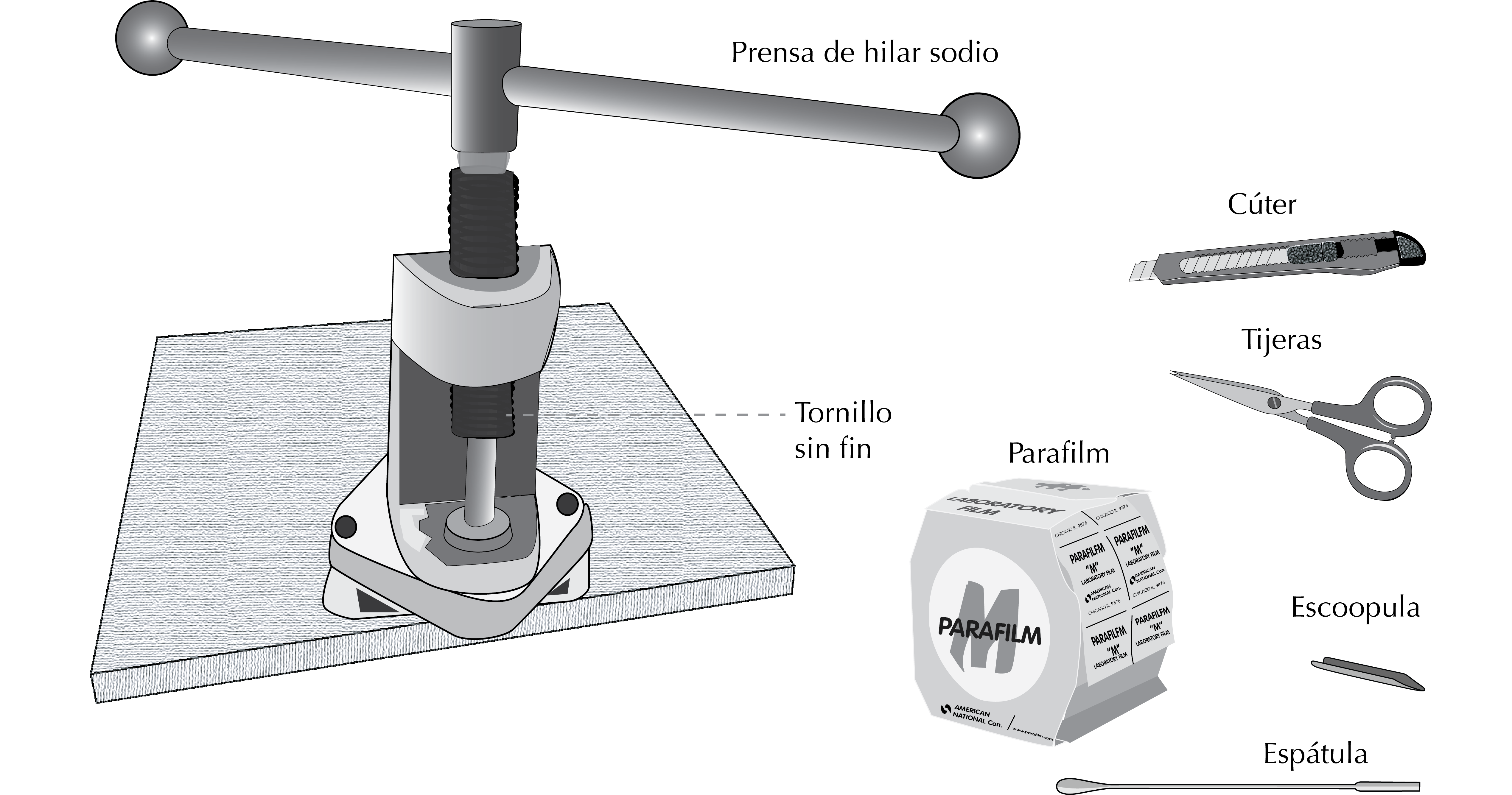
Spatula
Although they may differ greatly in size and shape, all are used for handling small amounts of solids. A special type of metal spatula called scoopula[5] is used for transferring solids to a weighing paper.
Scissors
There are several types of scissors, with their design depending on their use. Common scissors (office) are used, for example, to cut filter paper.
Parafilm®
It is a type of thermoplastic film, flexible and self-sealing that is sold in rolls and is used for closing containers or sealing glass or plastic plugs. It is resistant to most common reagents in a chemistry lab.
Cutter
It is a type of knife which features a blade with a very sharp and with a handle that can be plastic or metal. It is often used for cutting TLC plates, gum rubber or silicone tubing, corks, etc.
Sodium wire press
Sodium is a ductile, malleable metal, with a low melting point. Its highly reactive nature makes handling it relatively dangerous. It reacts violently with water and with low-molecular-weight alcohols, producing hydrogen. It is easily oxidized with air and covered quickly by a layer of oxide or carbonate of sodium, which is passive. In order to increase their reactivity in organic reactions, it is generally added to the reaction in the form of a very thin thread (to increase its surface).
It can be safely handled if cut into small pieces and compressed in a sodium press and collected in a container filled with an inert liquid sodium (hexane or toluene). Other than as reagent, sodium wires are often used to maintain anhydrous solvents after distillation.
It can be weighed in a container with a dry inert liquid, and the sodium are added in small pieces or in wire if available. Sodium wastes in the laboratory can be discarded by reaction with EtOH (never add water) in a fume hood and away from heat sources.
Video about Lab Tools and Equipment
References and notes
- [1] The term in English “laboratory glassware“refers to a set of glassware in the laboratory.
- [2] Taper of 10 %, corresponding to a semi-angle α of the conical surface matte 2º 51′ 45″ with a tolerance of ±2′.
- [3] The joints frosted is sometimes blocked (agarrotadas) after use. Many times you can unlock it by applying the solvents in the union while trying to light rotational movements of one part against another, you can also try to heat up, with an air dryer, a stove, or you can unlock by using an ultrasonic bath.
- [4] Caution with caps: Select the appropriate size, and we never use excessive force to close a container with a lid, the glass may break and produce cuts of importance in the fingers and hands.
- [5] Name registered by Thermo Fisher Scientific (from the English term “spatula-like scoop“), which has been generalized for the naming of this type of spatula.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2