What is Green Chemistry?
Chemistry is a science that makes an unquestionable contribution to improving the quality of life and the well-being of mankind, devising imaginative solutions in fields as diverse as the preparation of pharmaceuticals.
Thus, the discoveries carried out with chemistry have made it possible to fight effectively against a multitude of diseases.
In this sense, the development of phytosanitary products has provided crops to feed the world’s population, or the development of new materials that have improved the quality of life of many sectors of the population.
In spite of this, chemistry has a bad image in society, since it is common that in the media, the name chemistry/chemistry is synonymous with something dangerous, pernicious or negative.
The media often contribute to this idea by transmitting only the most negative aspects of this branch of science, such as the problems associated with the toxicity of some substances, or the problems of contamination derived from the improper use or inadequate handling of chemical products in general.
The so-called Green Chemistry deals with the design of chemical products and processes that reduce or eliminate the use and production of toxic or hazardous substances. It cannot be considered a branch of chemistry but rather a code of conduct to try to reduce the environmental impact of any chemical process.
Introduction
Faced with the challenges of developing better habits, and with the social awareness that arose around solving the problems derived from the use, storage or transport of chemical substances, the need arose in the 1990s to reorient some of the usual practices in teaching and research laboratories or in the chemical industry.
As a basis for the development of this new way of approaching chemical processes, the following documents and initiatives have been developed
- Documents UN on hazardous chemicals (1988).
- Pollution prevention act (1990) EPA.
- Project and grant funding program Presidential Green Chemistry Challenge of the US administration.
The Chemical Professional’s Code of Conduct of the American Chemical Society:
Chemical professionals should strive to do their work in ways that are safe and sustainable for the environment. This includes continuing to work to develop sustainable products and processes that protect the health, safety, and prosperity of future generations.
The so-called Green Chemistry is concerned with the design of chemical products and processes that reduce or eliminate the use and production of toxic or hazardous substances.
One cannot consider Green Chemistry as a branch of chemistry as is the case with the traditional classification of chemistry into Analytical Chemistry, Physical Chemistry, Inorganic Chemistry, Organic Chemistry and Chemical Engineering, but rather as a code of conduct that decreases the environmental impact of any chemical process, whether on a laboratory or industrial scale.
Synonyms for the concept of Green Chemistry are Sustainable Chemistry or Low Environmental Impact Chemistry.
Areas of activity
The main fields of action of Green Chemistry can be summarized in the following points:
- Use of alternative raw materials to the current ones, with lower toxicity and whose manufacturing processes present less environmental impact than the current ones, mainly based on renewable raw materials.
- Development of innocuous reagents to replace currently used toxic or dangerous reagents.
- Substitution of hazardous solvents for others that entail less risk in their use and handling.
- Development of alternative reaction conditions to the current ones, which consume less energy, shorten reaction times and simplify the isolation and purification of the final products.
Since the birth of Green Chemistry there has been an exponential growth in scientific publications of all kinds, giving rise to a multitude of specific journals, books and monographs related to the subject, as well as a multitude of networks and organizations of scientists interested in this field.
On the other hand, the postulates of Green Chemistry have been introduced in the curricula of chemists and chemical engineers as part of the subjects of the degrees, as specific subjects, postgraduate studies, etc., being nowadays a key subject in the training of future professionals.
12 Principles of Green Chemistry
Paul Anastas is considered the founder of Green Chemistry. In his book Green Chemistry: Theory and Practice he develops the so-called 12 principles of Green Chemistry.
- Prevention: It is preferable to prevent the production of a residue than to try to clean it up once it has formed.
- Atomic economics: Synthesis methods should be designed to incorporate as much as possible, in the final product, all the materials used during the process, minimizing the formation of by-products.
- Use of methodologies that generate products with reduced toxicity:Whenever possible, synthesis methods should be designed to use and generate substances that have little or no toxicity, both to humans and to the environment.
- Generate effective but non-toxic products: Chemicals should be designed to maintain efficacy while reducing toxicity.
- Reduce the use of auxiliary substances: The use of substances that are not essential (solvents, reagents to carry out separations, etc.) should be avoided as much as possible and, if they are used, they should be as innocuous as possible.
- Decreasing energy consumption: Energy requirements will be catalogued according to their environmental and economic impact, reducing them as much as possible. The synthesis methods will be carried out at room temperature and atmospheric pressure.
- Use of renewable raw materials: The raw material should preferably be renewable rather than exhaustible, provided that it is technically and economically feasible.
- Avoid unnecessary derivatization: The formation of derivatives (blocking groups, protection / deprotection, temporary modification of physical / chemical processes) should be avoided as much as possible.
- Potentiation of catalysis: The most selective catalysts possible, reusable as far as possible, will be used instead of stoichiometric reagents.
- Generate biodegradable products: Chemical products will be designed in such a way that at the end of their function they do not persist in the environment but are transformed into harmless degradation products.
- Develop analytical methodologies for real-time monitoring: Analytical methodologies will be further developed to allow real-time monitoring and control of the process, prior to the formation of hazardous substances.
- Minimize the potential for chemical accidents: Substances used in chemical processes shall be chosen to minimize the risk of chemical accidents, including fumes, explosions and fires.
Green Chemistry Goals
As an application of the principles and guidelines of “Green Chemistry”, the following objectives are intended to be achieved for any chemical process, either on an industrial or laboratory scale:
- Improve the use of available resources for the development of a chemical process.
- Reduce waste generated in any chemical preparation or handling process.
- To prepare materials by improved procedures that reduce undesirable effects on the environment.
- Substitute those reagents and products that due to their intrinsic toxicity are dangerous, for others that have the same properties and applications and cause less impact on the environment.
- Reduce the energy required to produce substances of interest, either by using much faster processes or by using renewable energies that entail a lower energy cost with the same efficiency.
- To reduce the overall toxicity or hazardousness of the substances needed to obtain a given compound and that of the compound itself.
- To reduce costs by eliminating any handling that is not strictly necessary and to reduce the time spent in the preparation of a substance.
- Promote all necessary actions to make chemistry compatible with sustainable development.
Parameters in Green Chemistry
The environmental impact of a chemical reaction can be quantified using a number of parameters. Among them, the E-factor and the atomic economy stand out.
- Factor E: One of the first parameters for the evaluation and analysis of the environmental impact of a chemical reaction was introduced by R. Sheldon. It is called the E-Factor. The concept is simple and easy to understand, applicable mainly to the industrial sector. It is calculated by dividing the total mass of waste produced in the preparation of a compound, by the total mass of product manufactured or synthesized. The lower the value of E, the lower the environmental impact of the process.
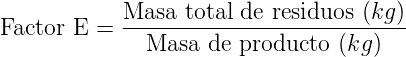
In Table 1 the average values of the E factor for various branches of the chemical industry are listed.
| Industrial sector | Production volume (Tons) | Factor E (kg waste / kg product) |
| Petrochemicals | 106-108 | < 0.1 |
| Chemical Industry | 104-106 | < 1-5 |
| Fine chemistry | 102-104 | 5-50 |
| Pharmaceutical Industry | 10-103 | 25-100 |
Atomic economy
The concept of atomic economics is due to B. M Trost. It is probably one of the most useful reaction analysis parameters, as it allows to evaluate the amount of waste generated in a reaction or sequences of reactions. Atom economy has exerted a great influence on the further development of organic synthesis as a whole, in that it focuses the design of synthetic procedures towards the concept of sustainability.
The calculation of the atomic economy makes it possible to quantify the use made of each of the atoms of a reagent by indicating which of them are actually incorporated into the final product. With the concept of atomic economy some approximations are assumed to simplify the calculations.
Since it is a measure of the extent to which the reagents are incorporated into the final product, the amount of solvent used, excess organic reagents, catalysts or inorganic salts that may be added to the reaction are not included in the calculation, nor is the reaction yield involved.
Examples
For a reaction A + B → C, the atomic economy will be:
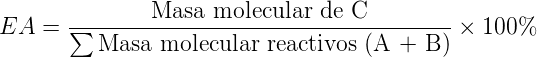
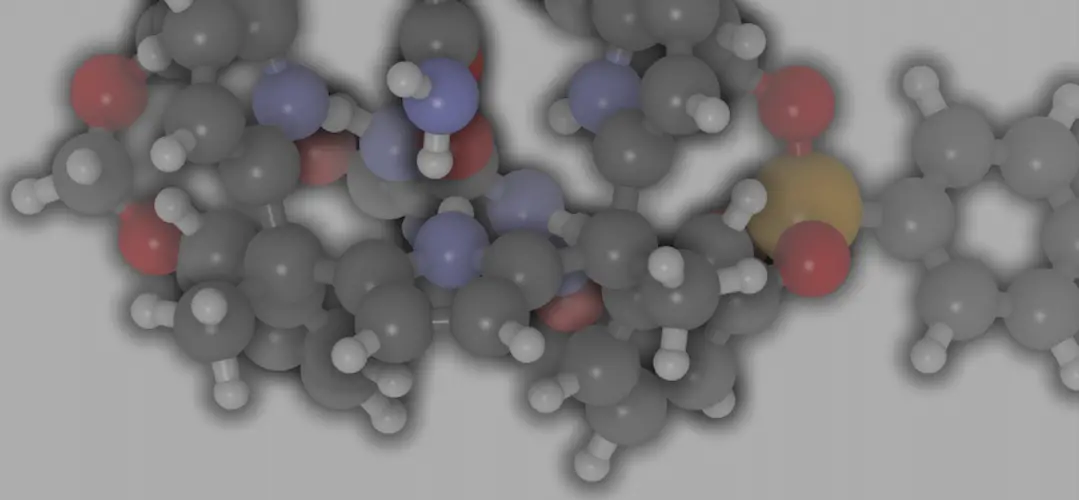
As an example for the calculation of the AE we can consider the preparation of ethylene oxide (oxirane) by the traditional method. Assuming a reaction yield of 100 %, the atomic economy of the process would be 25.46 %.
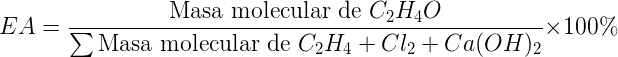
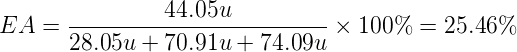
<Green Chemistry in teaching laboratories
The application of the principles of Green Chemistry to the teaching laboratories of Organic Chemistry can present two aspects.
On the one hand, in terms of their management, through an evaluation of the techniques used, the improvement of the safety of the processes with the available resources and the calibration of the toxicity of commonly used reagents and solvents.
On the other hand, and as a consequence of this evaluation, it is possible to progressively replace the most dangerous or harmful substances with others that are less aggressive with the environment and with people and that perform the same function. In addition, new techniques can be developed that, while maintaining or even increasing the level of training of the students, are more respectful of the environment.
In this sense, two types of actions can be carried out, in accordance with the trends followed in most universities and other educational centers interested in disseminating the principles of Green Chemistry in laboratories.
- Development of specific Green Chemistry practices, where alternative methods and procedures to the traditional ones are taught.
- Learning microscale techniques involves acquiring the same skills as conventional laboratory techniques with much lower reagent and solvent consumption and minimal waste generation for each experiment.