Go to the page with the solutions to the problems.
Alkanes – list of problems
Problem 1:
(a) Draw and name all possible isomers of molecular formula C5H12.
(b) Identify the primary, secondary and tertiary hydrogens in each of the structures.
(c) Deduce which isomer forms a single compound in the monobromination reaction, without considering possible stereoisomers.
Problem 2:
Draw the profile of the chlorination reaction via radical of methane, including the most likely transition state that would be observed in such a profile.
Problem 3:
The halogenation reaction via radical of propane and 2-methylpropane gives the products and percentages indicated. Justify these experimental results and determine the relative reactivities of each type of hydrogen as a function of the halogen used.
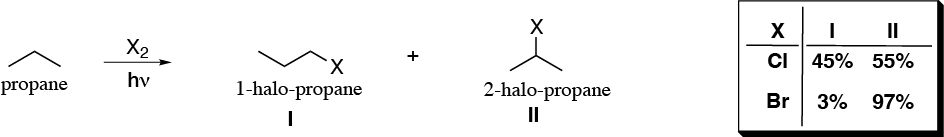
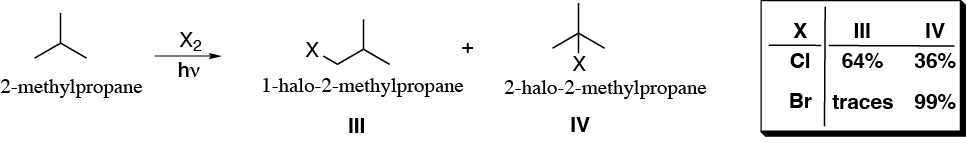
Problem 4:
In view of the results of problem 3, predict what proportion of products will be obtained in the monochlorination of 2,3-dimethylbutane.
Problem 5:
Assuming that the order of reactivity of the hydrogens in the bromination reaction is:
- 3º 1600
- 2º 82
- 1º 1
predict the major product in the monobromination reaction of propane, butane, 2-methylpropane, cyclopentane and methylcyclopentane.
Problem 6:
State the major product(s) of the monobromination of the following compounds:
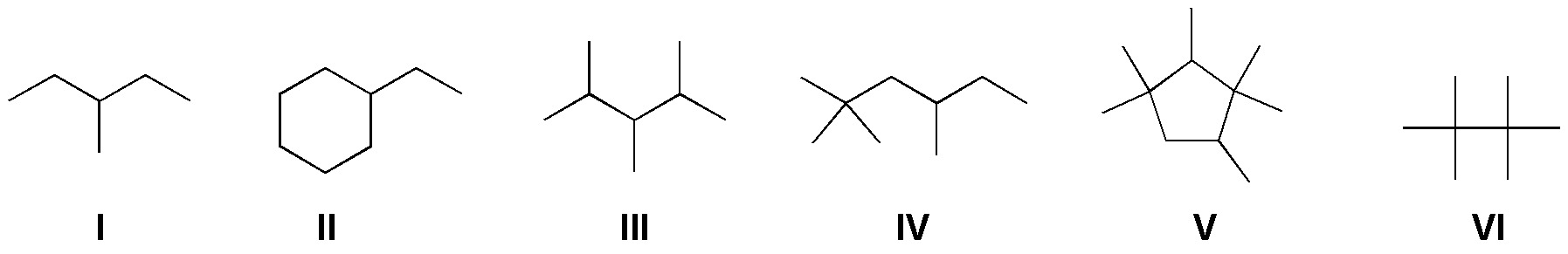
Problem 7:
Draw the constitutional isomers resulting from the monochlorination of 2,4-dimethylpentane, indicating which ones present chiral carbons.
Problem 8:
Assuming that the chlorination reaction of methylpropane with an excess of chlorine leads to the formation of a dichlorinated derivative, draw all the possible constitutional isomers that would be obtained.
Problem 9:
The bromination of pentane leads to the formation of three products one of which is a racemic form. Describe each of them and justify the result.
Problem 10:
Assuming in the racemic mixture of the previous problem a second bromination occurs, draw the most probable structure of the products obtained, justifying the result.
Problem 11:
Starting from (S)3-methylhexane, when the radical monobromination reaction is carried out, a reaction crude is obtained which is optically inactive. Justify the result obtained.
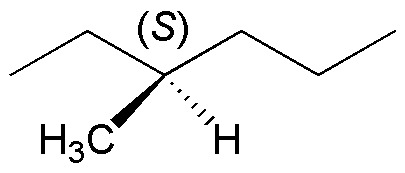
Problem 12:
If cyclobutane reacts with more than one mole of bromine, how many dibromocyclobutanes would be obtained?
Problem 13:
Draw the structures of the hydrocarbons (alkanes) that would be obtained in the pyrolysis of heptane.
Problem 14:
What are the products of complete combustion of n-pentane? Adjust the reaction.
Problem 15:
Adjust the complete combustion reaction of n-hexane.
Problem 16:
Adjust the partial combustion reaction of isooctane (CH3 )3CC(CH3 )3.
Problem 17:
Draw all the constitutional isomers (disregarding stereoisomers), obtained in the monochlorination of the following compounds:
(a) butane
(b) 2-methylbutane
(c) 2,3-dimethylbutane
(d) 3-methylpentane
Indicate in what proportion each of them would be formed taking into account the relative reactivities indicated in problem 3.
Problem 18:
Draw all the constitutional isomers, disregarding stereoisomers, obtained in the monochlorination of the following compounds:
(a) 2,2-dimethylbutane
(b) 2,2-dimethylpropane
(c) n-hexane
(d) methylcyclohexane
(e) 1,4-dimethylcyclohexane
Problem 19:
Identify the products obtained in the monochlorination of 2,4-dimethylpentane. Indicate whether any of these compounds have chiral carbons. If the rotational power of the reaction crude is determined, what would be the result?
Problem 20:
Identify the products obtained in the dibromination of 2,4-dimethylpentane. Indicate if any of these compounds have chiral carbons.
Problem 21:
Calculate the relative reactivity of the hydrogen atoms of butane in the chlorination reaction, knowing that monochlorination of this hydrocarbon yields 1-chlorobutane (28%) and 2-chlorobutane (72%).
Problem 22:
Calculate the relative reactivity of the hydrogen atoms of 2-methylbutane in the chlorination reaction, knowing that monochlorination of this hydrocarbon leads to the formation of 1-chloro-2-methylbutane (27%), 2-chloro-2-methylbutane (23%), 2-chloro-3-methylbutane (36%) and 1-chloro-3-methylbutane (14%).
Problem 23:
From the following relative reactivity data [ 1 : 1 : 4 : 6 ] of the following types of hydrogens [(CH3-)3 : (CH3-)2 : CH2– : CH- ], indicate the products obtained and the expected percentage in the monochlorination of 2,2,4-trimethylpentane.
Problem 24:
Predict what will be the major product(s) of the monobromination reaction for the following alkanes:
(a) methylcyclopentane
(b) 2-methylpentane
(c) 2,2,5-trimethylhexane
(d) methylcyclopropane
(e) isopentylcyclopentane.
Problem 25:
Assuming you have to prepare a monobrominated derivative by a radical reaction, which hydrocarbons would you choose from the following list, and which would you discard?
(a) methylcyclohexane
(b) 2-methylpropane
(c) 2,2-dimethylpropane
(d) 2,3-dimethylbutane
(e) 2,4-dimethylpentane
(f) cycloheptane.
Problem 26:
The bromination reaction of both 2-methylbutane and (R)-3-methylhexane leads in both cases to a reaction crude that is optically inactive. Justify the result.
Problem 27:
Starting from propane, pentane and 2,3-dimethylbutane, draw all the possible alkanes that can be formed by combination of the radicals obtained in a pyrolysis reaction.
Problem 28:
Adjust the complete combustion reaction of n-butane.
Problem 29:
Fit the partial combustion reaction (CO + H2O) of n-dodecane.