Written by J.A Dobado | Last Updated on May 2, 2024
Objective
To obtain the concentration of vitamin C in a fruit juice using two redox titration methods, with iodine (KIO3) and with I2.

Background
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that is essential for human health. It plays a crucial role in many biological processes, such as collagen synthesis, wound healing, and immune system function. The concentration of vitamin C in a fruit juice can be determined using a redox titration with iodine (I2), or alternatively with potassiun iodate (KIO3). This procedure can be used to determine the concentration of vitamin C in other fruit juice samples and in vitamin C tablets. The following reaction takes place:
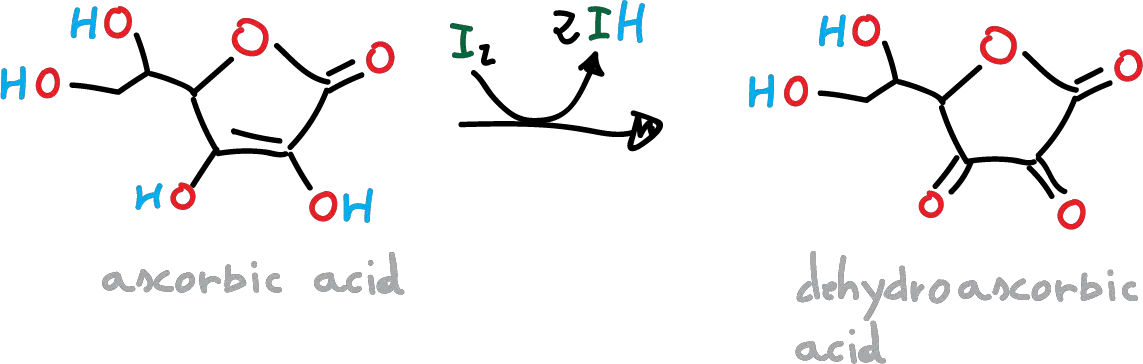
In the previous reaction, the ascorbic acid is oxidised to dehydroascorbic acid, and iodine is reduced to iodide ions. As long as ascorbic acid is present the iodine will be reduced to iodide ions, and as soon as there is no more ascorbic acid left the iodine will oxidize the starch that has been added as an indicator. The starch forms with iodine a blue-black starch-iodine complex, indicating the dead point of the titration.
The method using a standardized solution of iodine (I2) is more straigh forward than the alternative method using potassiun iodate (KIO3), however, due that KIO3 is most stable as primary standard than I2, the KIO3 method results more reliable.
Materials and Methods
- 0.5 g of ascorbic acid (standard)
- 5 mL of potassium iodide (KI) solution
- 5 mL of potassium iodate (KIO3) solution
- Standardized iodine (I2) solution (0.01 M)
- Fruit juice (e.g., orange, grapefruit, or lemon)
- 100 or 200 mL volumetric flask
- 250 mL conical flasks (Erlenmeyer)
- 10 or 20 mL Pipette
- 10 and 100 mL measuring cilinders
- Burette and stand
- Dropper bottle
- Starch solution (indicator)
- Beaker
Experimental procedure using I2
Prepare a standard solution of ascorbic acid by dissolving 0.5 g of ascorbic acid in a 100 mL volumetric flask with distilled water. Hereafter, prepare a fresh fruit juice sample by blending fresh fruit with water and straining the mixture.
Pipette 5 mL of fruit juice into a beaker. Fill the burette with the standardized iodine (I2) solution. Then, add a few drops of starch solution to the fruit juice in the beaker, and after that, add the standardized iodine solution to the beaker drop by drop, stirring continuously.
Continue the titration until the solution turns blue-black, indicating the end point. Record the volume of the standardized iodine solution used in the titration. Repeat the procedure three times and calculate the average volume of the standardized iodine solution used in the titration.
Starch indicator solution (0.5%): weigh 0.25 g of soluble starch and add it to 50 mL of near boiling water in a 100 mL Erlenmeyer. Stir to dissolve and cool to room temperature before using.
Iodine solution (0.005 mol/L). Weigh 2 g of potassium iodide (KI) into a 100 mL beaker. Weigh 1.3 g of iodine (I2) and add it into the same beaker. Add a few mL of distilled water and stirr for a few minutes until I2 is dissolved. Iodine solution is transfer to a 1 L volumetric flask, making sure to rinse all drops of solution into the volumetric flask using distilled water. Make the solution up to the 1 L mark with distilled water.
Data analysis
The concentration of ascorbic acid in the fruit juice can be calculated using the following equation:
C = (V * M) / (V0 * MW)
Where:
- C = concentration of ascorbic acid (mg/L)
- V = volume of the standardized iodine solution used in the titration (mL)
- M = molarity of the standardized iodine solution (mol/L)
- V0 = volume of the fruit juice sample (mL)
- MW = molecular weight of ascorbic acid (176.13 g/mol)
Experimental procedure using KIO3
First, prepare a standard solution of ascorbic acid by dissolving 0.5 g of ascorbic acid in a 100 mL volumetric flask with distilled water. Then, prepare a fresh fruit juice sample by blending fresh fruit with water and straining the mixture.
Pipette 5 mL of fruit juice into a beaker, add 5 mL of KI solution to the beaker and stir (magnetic stirring). Then, add 5 mL of KIO3 solution to the beaker while continuing to stir.
Fill the burette with the standard solution of ascorbic acid and add the standard solution of ascorbic acid to the beaker drop by drop, stirring continuously.
Continue the titration until the solution turns blue-black, indicating the end point. Moreover, add a few drops of starch indicator to the solution and stir. Continue the titration until the solution turns blue-black and the appearance of a blue-black complex indicates the end point. Finally, record the volume of the standard solution of ascorbic acid used in the titration. Repeat the procedure three times (to yield an average concentration value) and calculate the average volume of the standard solution of ascorbic acid used in the titration.
Starch indicator solution (0.5%): weigh 0.25 g of soluble starch and add it to 50 mL of near boiling water in a 100 mL Erlenmeyer. Stir to dissolve and cool to room temperature before using.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Ascorbic acid | 176.12 | 190-192 | 552.7 | 1.694 |
| Iodine I2 | 253.81 | 113 | 184 | 4.930 |
| Potato starch | - | - | - | 1.500 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Ascorbic acid | Non-hazardous |
| Iodine I2 |   |
| Potato starch | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Ascorbic acid | CIWBSHSKHKDKBQ-JLAZNSOCSA-N |
| Iodine I2 | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
| Potato starch |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2