Written by J.A Dobado | Last Updated on April 22, 2024
Objective
Use of fractional distillation for the purification of mixtures of liquid substances with close boiling points.
Background
Water and acetone are miscible so that the only way to separate them once mixed is by distillation. Fractional distillation is indicated when the difference in the boiling point of the liquids to be separated is approximately less than 20 ºC.
Moreover, in the presence of an acid or a base, water readily adds to the carbonyl function of aldehydes and ketones, resulting in the formation of a hydrate (geminal-diol or gem-diol) that exists in a reversible equilibrium. The term “geminal” or “gem” is derived from the Latin word “geminus,” meaning twin.
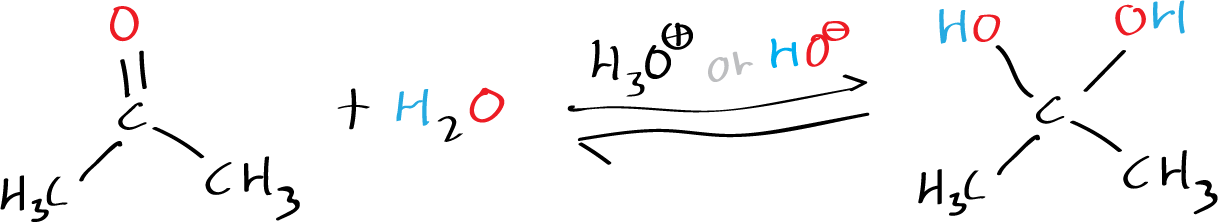
However, although in this case the difference between the e.g. of water and the acetone is greater, this technique will be used because it is very efficient in the separation. The efficiency comes from the incorporation to the assembly of a rectification column that behaves as if multiple single distillations were performed.
Experimental procedure
In a 250 mL round bottom flask 60 ml of water and 40 ml of acetone are added. In addition, to see clearly at the end of the process the separation of the two colorless liquids, several drops of a dye (bromophenol blue) will be added that will remain in the water and will give it a blue color. One or two boiling chips are also added so that the boiling is homogeneous.
To perform fractional distillation of an acetone/water mixture, you will need a fractional distillation setup, which typically consists of a distillation column, a heating source, and a condenser.
The fractional distillation setup is prepared, using a vigreux column and connecting the water inlets to the water condenser (note that the water inlet is upstream of the condenser). The heating will be carried out gently, so that a progressive and smooth increase of the temperature in the distillation head is observed when acetone vaporizes.
The vapor is then passed through a distillation column, which is a tube packed with small glass beads or other packing material (or in this particular case with a vigreux column). As the vapor rises through the column, it comes into contact with the packing material, which increases the surface area and allows for more efficient vapor-liquid exchange.
As the vapor cools and condenses, it passes through a condenser, which cools the vapor and turns it back into a liquid. The initial distillate fraction will be collected (in a test tube) at a temperature in the range of 56-62 ºC. The distillate corresponds to practically pure acetone. Approximately one drop of distillate per second is collected in the beaker. When the temperature of 62 ºC is reached or the original amount of 40 ml of acetone is obtained, the distillation process is stopped. The remaining liquid in the distillation flask will be mostly water.
It is important to note that the purity of the acetone obtained through fractional distillation will depend on the starting mixture and the efficiency of the distillation process. It may also be necessary to perform additional purification steps to obtain pure acetone. However, fractional distillation is a useful technique for separating the components of an acetone/water mixture. With the proper equipment and setup, it is possible to obtain relatively pure acetone through this process.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetone | 58.08 | -94 | 56 | 0.791 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetone |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| bromophenol blue | UDSAIICHUKSCKT-UHFFFAOYSA-N |
Video on separation of acetone and water through fractional and simple distillation
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A laboratory experiment on the boiling-point curves of non-azeotropic binary mixtures
George W. Bennett
Journal of Chemical Education 1929 6 (9), 1544
DOI: 10.1021/ed006p1544