Written by J.A Dobado | Last Updated on April 22, 2024
Nomenclature of linear alkanes
Linear alkanes are the simplest organic compounds from the structural point of view.
They are formed by unbranched chains of carbon atoms, with their respective hydrogens, linked by single bonds.
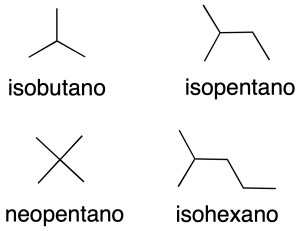
They are named by prefixing a Greek prefix indicating the number of carbons (see Table-A1 in the Appendix) with the suffix –ane (recommendation A-1.1). Some common names applicable only to hydrocarbons without substituents are retained (recommendation A-2.1).
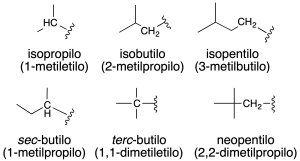
Monovalent radicals derived from alkanes are named by replacing the termination –ane by –yl (recommendation A-1.2) and are numbered starting from the carbon attached to the main chain which is taken as carbon 1.
| Name (Abbreviation) | Structure |
| Metyl (Me) | CH3– |
| Etyl (Et) | CH3CH2– |
| Propyl (Et) | CH3CH2CH2– |
| Butyl (Pr) | CH3(CH2)2CH2– |
| <Pentyl | CH3(CH2)3CH2– |
Also, some common names of radicals (recommendation A-2.25) are retained and are shown in the figure together with the systematic name in parentheses, and in the table.
| nº of C | Name | nº of C | Name | nº of C | Name |
|---|---|---|---|---|---|
1 | methane | 7 | heptane | 13 | tridecane |
2 | etane | 8 | octane | 20 | eicosane |
3 | propane | 9 | nonane | 30 | tricontane |
4 | butane | 10 | decane | 40 | tetracontane |
5 | pentane | 11 | undecane | 100 | hectane |
6 | hexane | 12 | dodecane | 200 | dichectane |
Nomenclature of branched alkanes
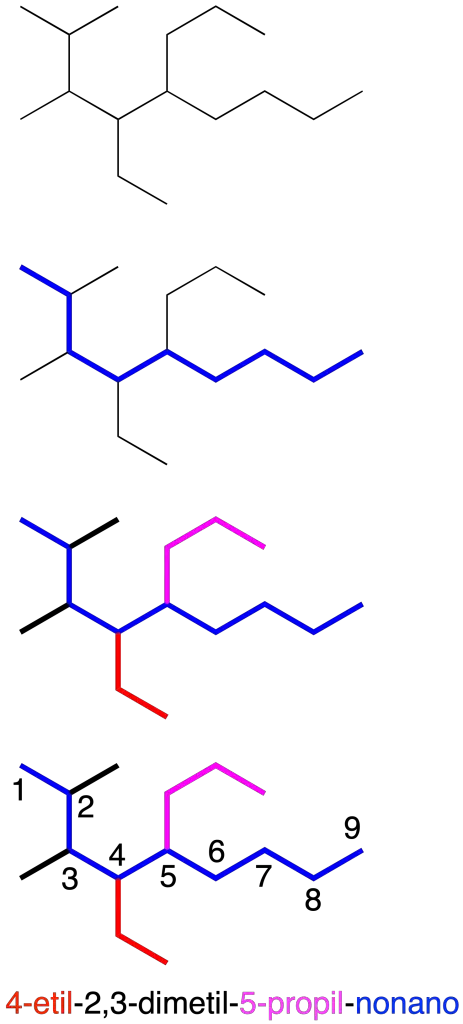
They are named on the basis of the carbon chain with the highest number of carbon atoms. Branches are considered as substituents. The positions of the substituents are indicated with Arabic numerals by means of locators (recommendation A-2.2).
To correctly name branched alkanes, it is convenient to follow the steps below:
- Locate the main chain.
- Identify the substituents using if necessary multiplier prefixes (di-, tri-, tetra-, penta-, …). In the case of substituents which themselves have branches, the name of the complex substituent is written in parentheses. In addition, in a radical the carbon directly attached to the main chain is numbered as carbon 1.
- Number the main chain, so as to obtain the lowest combination of locators.
- Alphabetically order the substituents without taking into account the multiplicative prefixes.
- Type the complete name preceding the locator, separating them from the text with hyphens. Use multiplicative prefixes for repeating substituents, indicating the locators separated by commas. Finish with the name of the main chain.
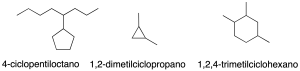
Nomenclature of monocyclic cycloalkanes
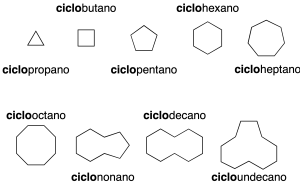
The simplest cyclic alkanes consist of a single cycle (monocyclic). They are named by prefixing the cyclo– prefix to the name of the acyclic alkane with the same number of carbon atoms (recommendation A-11.1) (see Table-1 in the Appendix).
Monovalent radicals derived from cycloalkanes are named by the –yl termination, with carbon number 1 being the one attached to the main chain (recommendation A-11.2).
In substituted cycloalkanes the same 3rd-step of branched alkanes is followed to assign the lowest locator combination.
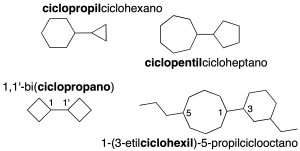
Nomenclature of polycyclic cycloalkanes
They are multi-ring systems and are further subdivided into three families of compounds:
Rings joined by a C-C single bond
They are systems of two or more cycles joined by a single (the simplest) or double bond.
The smaller ring is named as a radical ending in the name of the larger one (if it has substituents, these are ordered alphabetically as in branched alkanes). If the rings are the same, number the bridging carbons 1 and 1′, prefixing the bi– prefix to the ring name in parentheses (recommendation A-51).
One-carbon bonding (spiranes)
They are bicyclic compounds with a carbon atom common to both rings.
The simplest case is obtained when two rings are joined by a spiranic carbon. They are named with the prefix spiro– and using as suffix the linear alkane with the number of carbons equal to that of the system. Between the prefix and suffix, two numbers are specified in square brackets and separated by a dot, indicating the number of carbons in increasing order that are attached to the [n.m] spiranic carbon (recommendation A-41).

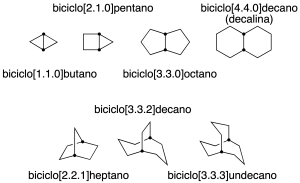
Rings sharing more than one carbon
They are polycyclic hydrocarbons with two or more carbon atoms common to two or more rings. These carbons are called bridgeheads.
The simplest case corresponds to bicycles. In this case, the name is constructed with the prefix bicyclo-, followed by 3 numbers in square brackets and in decreasing order. These numbers indicate how many carbon atoms are connected to the common vertices of the rings (in the following figure these carbons are highlighted). Finally, the name is terminated with the suffix of the linear alkane with the same number of carbons as the system (recommendation A-31.1).
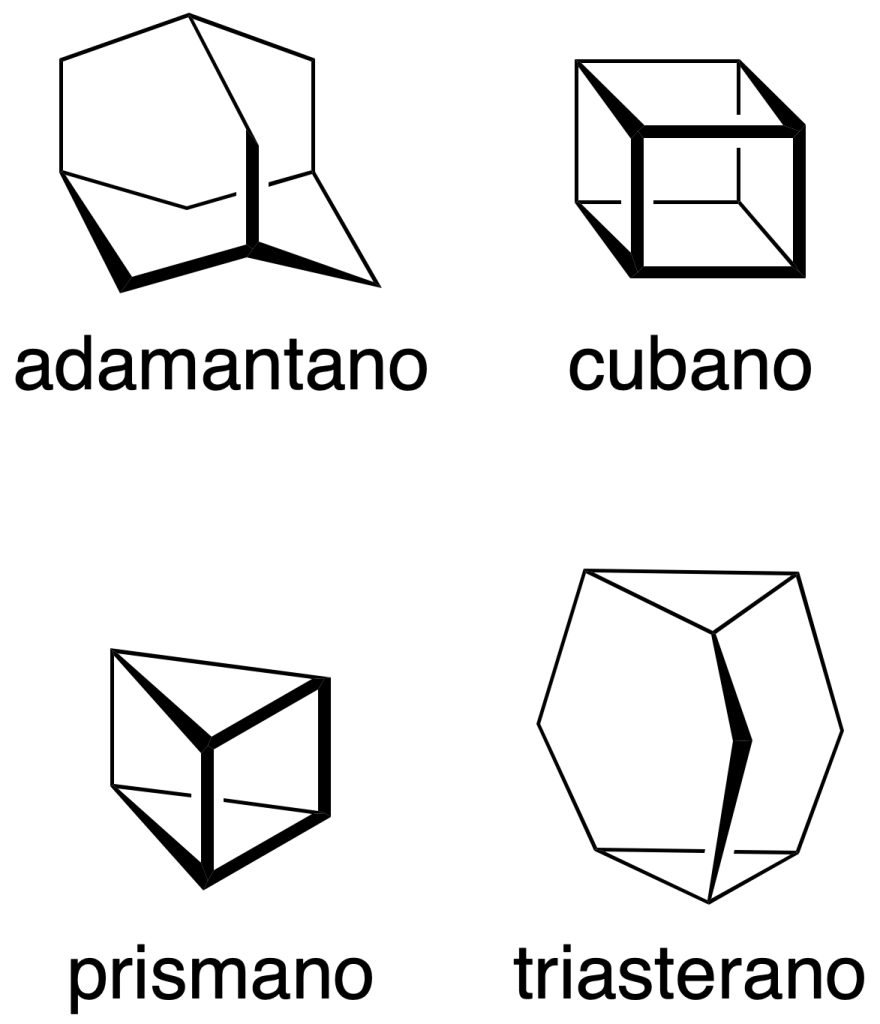
It should be mentioned that some common names are also preserved and are indicated in parentheses in the following figure:
Numbering
The numbering system on bicycles starts with the lowest locator on a bridgehead. After that, the largest ring is numbered. Next, the other bridgehead is numbered. Next, we continue with the intermediate ring. And finally, we finish with the smallest ring (recommendation A-31.2).
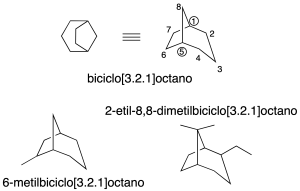
In addition, it should be noted that some non-systematic names have been retained, such as those shown in the figure:
Return to the page naming mono-functional compounds.