Written by J.A Dobado | Last Updated on April 22, 2024
These are hydrocarbons containing at least one C=C double bond. They are named by changing the -ane termination of the corresponding alkane (IUPAC recommendation A-3.1) or cycloalkane (recommendation A-11.3) to -ene. The first term in the series is ethene, and its common name is ethylene (CH2=CH2).
Common names such as allene (CH2=C=CH2) or isoprene (H2C=C(CH3)CH=CH2) are preserved. The stereochemistry of the double bond is specified in parentheses at the beginning of the name (E or Z; E= trans substituents and Z= cis substituents).
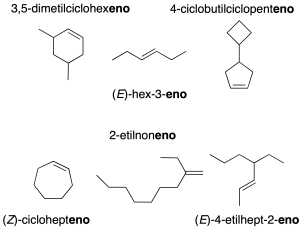
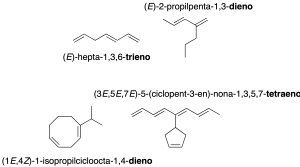
When there is more than one double bond, the corresponding multiplicative prefix di-, tri-, tetra– … is used (recommendation A-3.3). If there are branches, the longest chain containing the largest number of double bonds is taken as the main chain (recommendation A-3.4).
Alkene-derived radicals are named by replacing the –ene termination with –enyl in both cyclic (recommendation A-11) and acyclic (recommendation A-3.5) compounds.
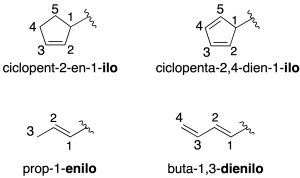
Some common radical names (recommendation A-3.5) such as vinyl (CH2=CH-) and allyl (CH2=CH-CH2-) are preserved.
Return to the page naming mono-functional compounds.