Written by J.A Dobado | Last Updated on April 22, 2024
Formally, amines can be considered to be derived from ammonia by substitution of one, two or three hydrogens by alkyl radicals. They are classified into primary amines, RNH2, secondary, RR’NH, and tertiary, RR’R “N (recommendation C-811.2).
Primary amines
Primary amines of general formula RNH2, can be named by two procedures:
- Substitutive nomenclature: the name is constructed from the name of the hydrocarbon with the same number of carbons ending in amine.
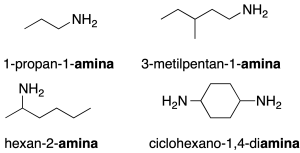
- Radical-function nomenclature: the substituent attached to the -NH2 group is indicated as a radical, followed by the word amine. Secondary and tertiary amines are named using the nomenclature radical function. (recommendation C-813.1).
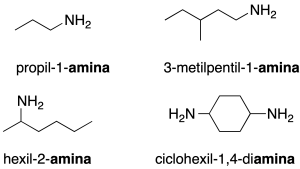
In case of doubt and for any type of amine, the radicals attached directly to the nitrogen are prefixed with N-. If there is more than one nitrogen, they are distinguished with (N, N’, N”…) (recommendation C-814.2). When the -NH2group acts as a substituent, it is designated xxx. If there is more than one group, the multiplicative prefixes (di-, tri-, tetra-, …) are used (recommendation C-813-2).
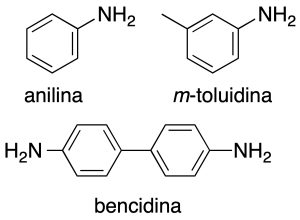
Some common names are preserved, especially in arylamines:
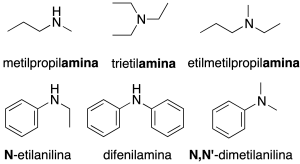
Secondary and tertiary amines
Secondary amines, RR’NH, and tertiary amines, RR’R “N are assigned the name of the substituents attached to the nitrogen atom using multiplicative prefixes (if necessary) ending in the word amine. (recommendation C-814.4). Another possibility is to name them as N-substituted (on the nitrogen) derivatives of a primary amine (recommendation C-814.1).
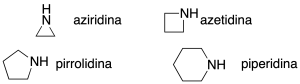
The 3-, 4-, 5- and 6-linked cyclic secondary amines are given the common names shown in the figure (recommendation B-2.11). The heteroatom retains the locator 1.
Ammonium salts
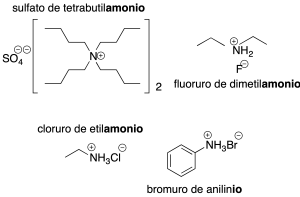
In ammonium shals, the nitrogen atom is bonded to four substituents and therefore has a formal charge of +1.
They are generally named by indicating the nitrogen substituents ending in the word ammonium, preceded by the anion.
For commonly named amine derivatives, salts are named by eliminating the final vowel and substituting the ending –ium(recommendations C-816.1 and C-861.1).
Return to the page naming mono-functional compounds.