Written by J.A Dobado | Last Updated on April 22, 2024
The nucleus is the central portion of the atom that contains protons (positive particles, p+) and neutrons (neutral particles, n0). Around the nucleus revolve electrons (negative particles, e–). An atom can be characterized by its atomic and mass numbers. The atomic number (Z) corresponds to the number of protons contained in its nucleus and identifies the element. The mass number (A) is the sum of the number of protons and neutrons, according to the expression:
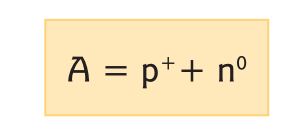
As we can appreciate with the above expression, the mass number increases as the number of protons increases, which also leads to an increase of neutrons in the nucleus in order to provide stability. Finally, it should be noted that elements with an atomic number greater than 83 are considered radioactive.