
Definition of Organic Chemistry
It is common to observe how the progress and development of science and technology, to a great extent unpredictable, can cause the overflowing of the limits of definitions and concepts that saw the light not many years ago, and which, at the time of their birth, seemed to enjoy some bases or pillars of support, as well as some limits, concrete, immutable and perpetual.
In the field of Chemistry itself, its first definition as a science that studies the transformation of matter, based on the most fundamental definition of the atom as an indivisible entity, has had to undergo modifications in order to specify its frontier with Atomic Physics, as a consequence of the subsequent discovery of the divisibility of the indivisible atom.
Organic chemistry is no exception to this trend. Nowadays, it is impossible to define its concept in a definitive way, since over the years the clear and continuous dividing line that has emerged for two centuries, dividing Chemistry into two main branches: Inorganic and Organic, has deteriorated profoundly, as a consequence of the increasingly frequent mutual intrusions.
To such an extent that today we can speak of a broad common area in which organic and inorganic chemists work together. A frank zone, with its own products and reactions, which cannot be clearly and conclusively squared in one of the two initial fundamental branches.
The definition of organic chemistry as the chemistry that studies the structure and reactions of organic compounds, although semantically correct and congruent, nevertheless becomes conceptually imprecise and reiterative, but it is the only valid definition capable of adapting to the real absence of concrete limits, as long as it clearly defines what is and should be understood as an organic compound.
However, the concept of “organic compound”, based on this definition, has not escaped the sweeping progress of science and does not correspond to its natural and primitive meaning.
Therefore, it is now applied by chemists as the result of a more or less universal consensus or feeling that is born in their conscience, modeled by their greater or lesser experience in this field. Logically, this organic consciousness has evolved parallel to the development of this science, so a brief consideration of this historical development can help and clarify the understanding of its essence.
Historical development
The Figure shows schematically the genealogical tree of modern chemistry. Consequently, it shows the successive influences and interactions over several millennia. Likewise, the different cultures have exerted step by step in the evolution of knowledge until reaching Chemical Science.
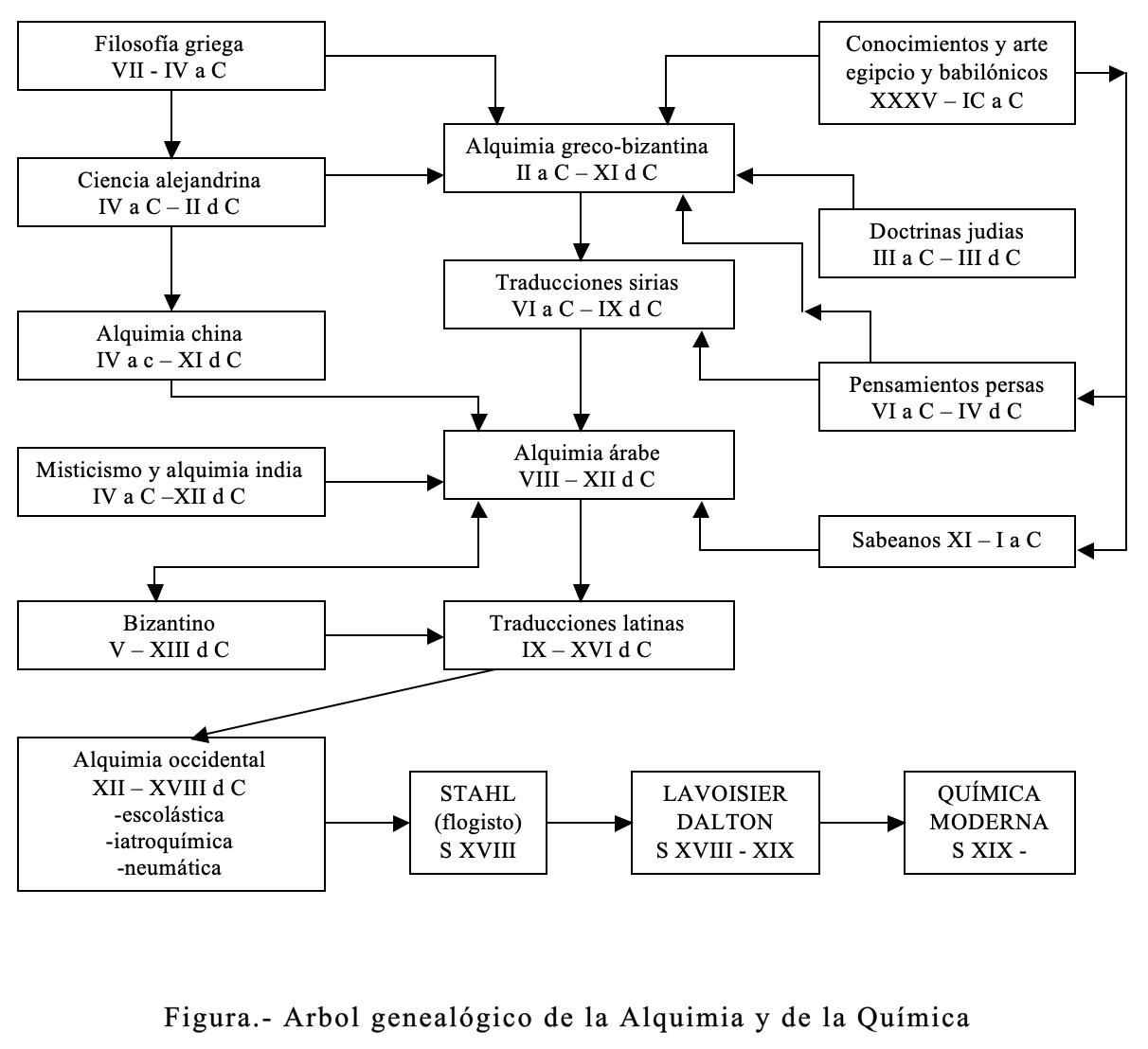
Some of the products and processes that today are considered typically organic, such as soap making, dyeing with purple, blonde or indigo, the fermentation of grapes in the manufacture of wine, or the preparation of perfumes, were already known according to Pliny by the Germans and Gauls.
However, such processes cannot be considered as properly scientific, but rather as simple rudimentary and primitive technological processes.
Until the 18th century, the interest of chemists was directed mainly towards the simplest inorganic problems. However, the number of known substances of animal or vegetable origin made it possible to see that in their behavior and composition they were very different from substances of mineral origin.
Thus, N. Lemery was mainly concerned with the study of salts extracted from vegetables and poisons, and in 1675 he published his course de chimie. In this book, he divided the compounds of natural origin into minerals, vegetables and animals.
This classification of compounds according to their origin was maintained for a whole century. In 1977, thanks to the experiments carried out by A.L. Lavoisier on the elemental composition of substances of animal and vegetable origin, T. Bergmann proposed to divide chemistry into two independent and, in his opinion, unconnected branches.
Consequently, one branch would deal with the study of substances of mineral origin and the other with substances of vegetable and/or animal origin. In this way, Bergmann drew that clear and defining line between the two parts of chemistry.
The study of substances of mineral origin already responded to a set of chemical laws of ordinary operation, while on the contrary, in the other branch of Chemistry, preparations and transformations were largely conditioned by the existence of a vital force of unknown nature called “vis vitalis”.
It was in 1806 that Bercelius finally used, for the first time, the term organic products to designate the compounds isolated from living beings. His treatise on Theoretical Chemistry (1817) includes among its volumes, one dedicated to the Chemistry of Organic Compounds. Finally, in 1827, Berzelius published his first treatise on organic chemistry.
The progressive application of Lavoisier’s techniques of quantitative elemental analysis soon made it possible to observe that all substances isolated from living beings contained the element carbon in their composition. This fact, however, did not receive the importance it should have, perhaps for the simple reason that a series of substances of mineral origin were already known that also contained it but did not behave like the former.
Gradually, a series of additional characteristics, such as their low melting and boiling points, combustibility and instability to heat, or the slowness of their reactions, supported and justified the division made by Bergmann.
However, this division soon became untenable, mainly as a consequence of the work of J. von Liebig and F. Wöhller. The former by demonstrating the validity of stoichiometric laws in the field of organic chemistry and the latter, by the preparation in 1824 of oxalic acid by hydrolysis of cyanogen and later in 1828 by his well-known synthesis of urea from ammonium cyanate, demonstrating the feasibility of the synthesis of organic compounds in the laboratory and from typically inorganic substances. This last achievement, in particular, demolished the whole edifice of the life-force theory.
Thus Liebig wrote in 1837, “the extraordinary and partly inexplicable production of urea without the assistance of vital functions, for which we are indebted to Wöhller, must be regarded as one of the discoveries with which a new era of Science begins.”
In spite of the impressive blow received, the vital force theory would stubbornly resist for 10 more years, contributing to its final downfall the saponification reaction of hydrocyanic acid with formic acid release, carried out by Eeloze in 1831, the total synthesis of acetic acid by Colbe (1845) and the contributions of other chemists such as Frankland and Berthelot, with the realization of numerous total syntheses.
With the definitive collapse of the vital force theory, a new era in organic chemistry was indeed opened, but at the same time the serious problem of justifying and redefining the scope of the division of chemistry into two branches, organic and inorganic, arose.
While it is true that the fundamental pillar that was obtained justified the independent existence of Organic Chemistry, had definitely cracked and collapsed, other initially small and secondary pillars seemed to have been strengthened enough to continue to support the Organic building.
In other words, organic chemistry was no longer the chemistry of living compounds and their life force, but organic products still contained the element carbon in their composition and were still characterized by a number of properties, which more than justified their independent study.
However, not content and satisfied with this justification, the post-vitalist era was characterized to a large extent by successive attempts to reinforce this organic edifice by means of a new pillar of an experimental theoretical nature. More specifically, of a constitutional theoretical nature.
The first of these attempts coincides with the birth of the old radical theory, which held that organic substances were made up of radicals, these being characteristic atomic groupings with a behavior similar to that of the atoms of inorganic chemistry, thus maintaining their integrity in the reactions in which they were involved.
Of this genre is the Theory of the “Etherines” of Dumas and Boullay, soon surpassed by the more consistent one of Dumas and Liebig, who in 1837 and in a joint communication affirmed: “Organic Chemistry possesses its own elements which as soon play the role of chlorine or oxygen as that of a metal. The cyan, amide, benzoyl groups, the radicals of ammonia, fats, alcohols and their derivatives, constitute the true elements of organic nature, while hydrogen and nitrogen only appear when organic matter is destroyed”.
This theory, taken too much to the letter, quickly led to serious contradictions, being replaced by a whole series of new more or less curious theories that failed to hold for a long time for one reason or another.
It was finally Wurtz, Gerhartt and Williamson in 1850 who, with the so-called “new group theory“, achieved a major advance in the theoretical order. These authors proposed the types water, ammonia, hydrogen sulfide, etc. as the basis of organic systematics in close connection with the radical theory.
From all the above, with the introduction by Kekulé in 1857 of the methane type and the establishment of the theory of the saturation capacity of the elements (similar to the valence theory) by Frankland in 1853, allowed Kekulé to arrive in 1859 at the establishment of the structural theory. Thus, the structural theory was finally able to give a satisfactory explanation of most of the known compounds from a structural point of view. Consequently, all this led to a clearer differentiation of this branch of chemistry.
The problem of benzene remained unresolved for several years, and after several theories to explain its structure, it was solved by Kekulé in 1865, who proposed the cyclic hexagonal structure with alternating double bonds.
It can thus be said that Kekulé’s structural theory is equal in importance to Wöhller’s synthesis of urea, as it fills the gap left by the old theory of the vital force. With it, organic chemistry becomes the chemistry of carbon and justifies its independence by the particular capacity of this element to combine with itself, which leads to the number of known organic compounds being many times greater than the number of known compounds of all the other elements. This proliferation of organic compounds, together with the use in their study of working methods very different from those employed in the rest of Chemistry, justifies, from a practical point of view, the convenience of maintaining this division.
As a consequence of structural theory, it was possible to explain the concept of isomerism. In addition, the concept of functional group was coined, as characteristic groupings of atoms within a molecular structure, which gives them certain properties. The concept of functional group allowed the classification of organic compounds according to the atomic groupings present, and the correlation of the properties of each series with the structure of the minimums, in short, the systematization of organic chemistry.
Despite the usefulness of structural formulas, they could not explain some facts such as optical isomerism, in fact, in 1815 J.B. Biot had discovered that some organic compounds in solution deflected the plane of polarization of a polarized light beam. In 1848 L. Pasteur observed under the microscope two types of crystals of sodium ammonium tartrate which he was able to separate. Their aqueous solutions showed rotational powers with different signs, a fact that was attributed to a structural symmetry that could not be explained by planar structural formulas.
In 1874 the structural formulas of Kekulé and Cooper acquired three-dimensional form, when J. H. Van’t Hoff and J.A. Le Bel independently but simultaneously demonstrated that the four bonds of the carbon atom were directed towards the vertices of a regular tetrahedron with the carbon in the center. The basis of stereoisomerism was thus established and an important stage in the knowledge of the three-dimensional structure of organic compounds began, of which I will highlight some interesting contributions. V. Meyer applied the three-dimensional structure to the nitrogen atom and W. Pope extended it to sulfur and selenium. Meyer also showed that although atoms rotate freely around a single bond, the presence of bulky groups can prevent this rotation. This is known as steric hindrance.
In the field of Stereochemistry we must highlight the work of E. Fisher, Nobel Prize winner in 1902, who assigned the configuration of each of the chiral centers of glucose, later completed by W.N. Haworth, Nobel Prize winner in 1937, with the cyclic hemiacetal structures; however, the term chirality was not introduced until the beginning of this century by W. Kelvin.
The studies carried out on the structures of cyclic compounds served as a basis for the subsequent development of conformational analysis. Thus in 1885 F.W.A. von Baeyer, Nobel Prize winner in 1905, introduced the theory of cycle stresses which explained the stability of 5- and 6-membered cycles.
In 1890 H. Sachse proposed two non-planar and flexible forms for cyclohexane, which were confirmed in 1920 by J. Boeseken and culminated in the differentiation between axial and equatorial substituents in 1950 by D.H. Barton for his contribution to Chemistry based on conformational analysis Barton was awarded the Nobel Prize in Chemistry in 1969.
Parallel to the knowledge about the structure of organic compounds, the study of the reactivity of organic compounds and organic synthesis began. At the end of the 19th century, some organic syntheses of aromatic compounds were carried out, in which the starting point was the most related aromatic compound and the synthesis consisted of the introduction or modification of some of the substituents. From then on, organic synthesis began to develop, having as its main objective the confirmation of the structures of the many compounds isolated from natural sources.
There are many syntheses carried out at the end of the last century and the beginning of this one, as well as many chemists remembered with the name of the reaction they developed. By way of example we will mention: syntheses such as glucose (E. Fisher, 1890), camphor (K. Komppa, 1903), tropinone (R. Robinson, 1917), hemin (E. Fischer, 1919), quinine (R. B. Woodward, I.W. Doering, 1944) or cortisone (R. B. Woodward, R. Robinson, 1951); and scientists such as Hoffmann, Grignard, Friedel, Crafts, Sandmeyer, Beckmann or Claisen.
In spite of these advances in the knowledge of organic compounds, at the beginning of this century the nature of the bond that supported them was still unknown. From the discovery of the electron in 1897 by J.J. Thompson, the Rutherford and Bohr atomic model was developed, which made possible the establishment of bond theories. It was in 1916 when K. Kossel and G.N. Lewis proposed two types of bonds: ionic and covalent. Lewis’ idea of covalent bonding between pairs of atoms that can complete their electron shells by sharing electron pairs was the basis for explaining the properties of most organic molecules. And because of its simplicity it is still used today for qualitative purposes.
However, some properties of organic compounds cannot be explained without a deeper understanding of atomic and molecular theory. They could therefore not be explained until the development of quantum mechanical theory, which developed during the 1920s-1930s. In 1924 L. de Broglie postulated the existence of a wave associated with the electron, laying the foundations of Wave Mechanics, which would have its formulation in the Schrödinger equation in 1926. On the other hand, W.K. Heisenberg in 1927 proposed the uncertainty principle while developing matrix mechanics, which together with Wave Mechanics constituted Quantum Mechanics.
This Quantum Mechanics could be applied to the hydrogen molecule and the energy of the bond could be calculated, which was in agreement with the known experimental value. The results thus obtained were interpreted in the sense that the formation of the H-H bond was due to the quantum mechanical phenomenon of a pair of shared electrons. This interpretation, which is in agreement with the Lewis theory, explains the rapid acceptance of this theory when a physical interpretation of the nature of the covalent bond was considered to have been achieved.
The development of these ideas by L. Pauling culminated in the formulation of the Valencia Bond method. This theory is based primarily on the use of orbitals located between the bonded atoms which, according to the Pauli exclusion principle, could only be occupied by two electrons with antiparallel spins. This theory is still valid for organic compounds that can be represented by a single Lewis formula.
However, in other compounds such as benzene, several simultaneous formulas were required for their representation. The wave function of these systems was obtained by combining the one corresponding to each individual formula as a mathematical expression of the resonance phenomenon. This phenomenon cannot be considered as a quantum-mechanical process but as the result of the particular use of classical formulas for obtaining the wave function of the system. Furthermore, the development of the theory of hybrid orbitals explained the directional character of the bonds in the tetrahedral structure of methane as well as the planar and linear structures in ethylene and acetylene.
In 1930, F. Hund, R.S. Mulliken, N.J. Leonard and E. Jones developed a second quantum-mechanical method called the Molecular Orbitals (MO) method. This theory considered the orbitals as delocalized systems in the whole molecular region being the molecule as a polyelectronic atom but at the same time multinuclear. In reality it is intended to solve the problem of a multielectronic system by solving N monoelectronic problems. The main difficulty of the theory lies in the difficult representation of these electronic distributions in simple structural formulas, especially in molecules with true electronic delocalization and without symmetry.
All the above has led to introduce some modifications in the MO theory the next step is the expansion in basis functions or as at first called the LCAO approximation represents each molecular orbital as a linear combination of atomic orbitals. Subsequent simplifications of the MO procedure led Hückel to obtain the Hückel method used at first for π-type systems and later extended to π- and σ-type systems in the Hückel-extended method. These procedures allow a molecular description of approximate complex systems to be carried out quite easily.
With MO theory it is possible to explain not only the way in which atoms in organic molecules are bonded together, and therefore their geometry, but also their reactivity and reaction mechanisms. At this time H. Lapwort, R. Robinson and C. Ingold began studies of reaction mechanisms. They introduced the new concepts of inductive effects and mesomers and tried to interpret organic reactions in terms of electron shifts from one point of the molecule to another.
The development of bonding theories is the basis of Theoretical Organic Chemistry in both its static (structural) and dynamic (reaction mechanisms) aspects.
Having said all the above, and to highlight the importance of organic chemistry and its involvement in society, we can give the following data: more than 95% of known chemical substances are carbon-based and more than 50% of current chemists are organic chemists. On the other hand, this discipline is involved in practically all facets of life, particularly in medicine and biochemistry or the synthesis of drugs, but also in other areas such as agriculture (pesticides or insecticides) materials industries (plastics, dyes, detergents, paints) cosmetics and perfume industries, petrochemical industry, etc.
In view of the above, it is not surprising to note the frequency with which the Nobel Prize in Chemistry has fallen into the hands of organic chemists: from E. Fischer in 1902 to R.F. Curl, H.W. Kroto and R.E. Smaley in 1996, 24 times the Nobel Prize in Chemistry has been awarded to organic chemists.
In view of all this, it is difficult to treat organic chemistry only as a science that deals with the structural study and reactivity of carbon compounds and derivatives, but rather, given its involvement in many facets of life, it is more than a science, it is an indicator of the level of progress of a country and its knowledge leads to understanding many factors on which the welfare of a society depends.
On the other hand, and together with its development and progress that continues at present, the degree of specialization reached in many of its different lines of action, has generated different fields that present entity by themselves, as areas of science, although in a general approach, all of them are included in Organic Chemistry. Thus, we can talk about Organometallic Chemistry, Biorganic Chemistry, Polymer Chemistry, Computational Organic Chemistry, etc.
State of the art of organic chemistry
As indicated in the previous section, it is clear that the classic frontiers of organic chemistry are becoming more and more scattered and that the mutual influence with other areas of knowledge is increasing significantly. Let us consider, for example, some of the new disciplines whose titles include terms classically associated with Chemistry: Molecular Biology, Molecular Genetics, Molecular Engineering, etc. Or in the opposite direction, importing terms clearly coming from other areas: Computational Chemistry, Chemical Ecology, Environmental Chemistry, etc.
Some of this is due to the progress that Organic Chemistry is making in certain directions such as, on the one hand, the attempt to rationalize, from an organic chemical point of view, many of the essential processes that are part of traditionally close disciplines (Biology, Medicine…) and on the other hand, the attempt to incorporate, as powerful tools for the study of organic chemical processes, advances achieved by other areas of knowledge (Computer Science, Mathematics, Physics…). All these aspects must be taken into account when configuring the contents of the teaching of our area.
Mutual influences between the different areas of chemistry
Also within chemistry, the boundaries of organic chemistry are shifting due to the high degree of overlap with the other areas. Let us look, for example, at the mutual influences between the different areas of chemistry in three fundamental and classical aspects of the domain of organic chemistry as shown in Figure.
- Purification and structural elucidation of molecular entities. Approach to the classical activities of Analytical Chemistry.
- Study of molecular reactivity from the point of view of both kinetic determinations and molecular modeling. This brings us closer to the knowledge of the mechanisms that regulate organic reactions, to the traditional Physical Chemistry.
- Chemical Synthesis allows us to connect with Inorganic Chemistry through the study of organometallic compounds. In addition, with the science of materials as regards the study of polymers. Also, with biochemistry through the study of biocatalyzed and biomimetic processes. With chemical engineering through the scaling up of synthetic processes and the use of new catalysts, etc.
All of the above, far from any attempt to expand by appropriation of areas traditionally belonging to other fields, whose didactic-organizational consequences are beyond our scope, should make us reflect on how to incorporate into our classic curricula the contents of the interfaces with other areas we have mentioned.
Thus, we can conclude that the extraordinary progress of organic chemistry in the last decades is a consequence of the incessant advance in several fields that have given theoretical support to this science, has extraordinarily improved its analytical power and has addressed new aspects of the reactivity of organic compounds.
Specifically, reference is made to the progress of the methods of isolation of organic compounds and their structural analysis, the development of Theoretical Organic Chemistry, and the progress of Organic Synthesis listed below:
| Structural determination |
| Reaction mechanisms |
| Theoretical Organic Chemistry |
| Organic Synthesis |
Bibliographic searches in Organic Chemistry
It should be pointed out that a rapid, reliable and as exhaustive as possible search for the results generated by other research groups is essential for the development of scientific activity.
In this sense, it is important to be familiar with the bibliographic search and know how to find, using the information in a bibliographic reference.
Like all other scientists, organic chemists have at their disposal a multitude of specialized literature that is increasing significantly. Thus, the volume of the literature in the area of organic chemistry doubles every 10 years and, even more seriously, diversifies into a multitude of new, highly specialized journals.
As a result, more and more time is required for searching for information and less and less time is left for reading and research. For more information see bibliographic sources in Organic Chemistry.