Written by J.A Dobado | Last Updated on April 22, 2024
Basic concepts
Heterocyclic compounds are cyclic compounds containing one or more non-carbon atoms in the ring structure. The most frequent elements are N, S and O. They are widely found in nature. Many of them are of fundamental importance to living systems; they can be key components in biological processes. For example: chlorophyll and the heme group are derivatives of the porphyrin system; the bases of nucleic acids are pyridine derivatives. Several vitamins (B1 , B2 , B6, … etc.) are heterocyclic compounds.
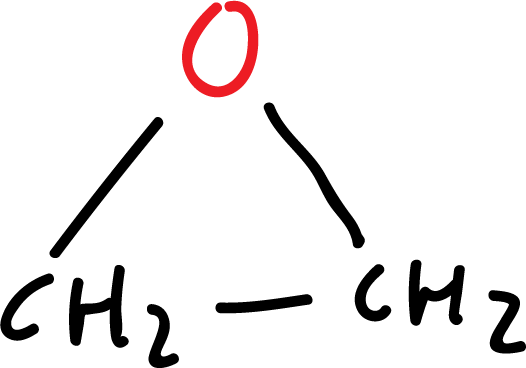
The simplest heterocycles are composed of three-membered rings:
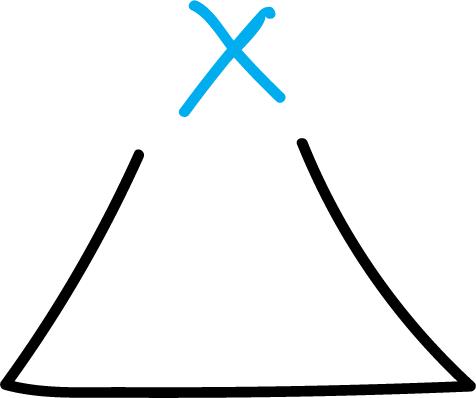
followed by 4, 5, 6, 7, etc. up to higher heterocycles.
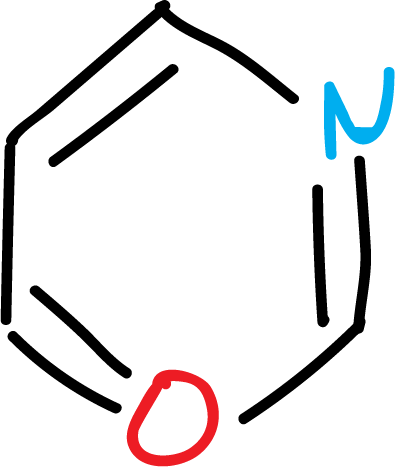
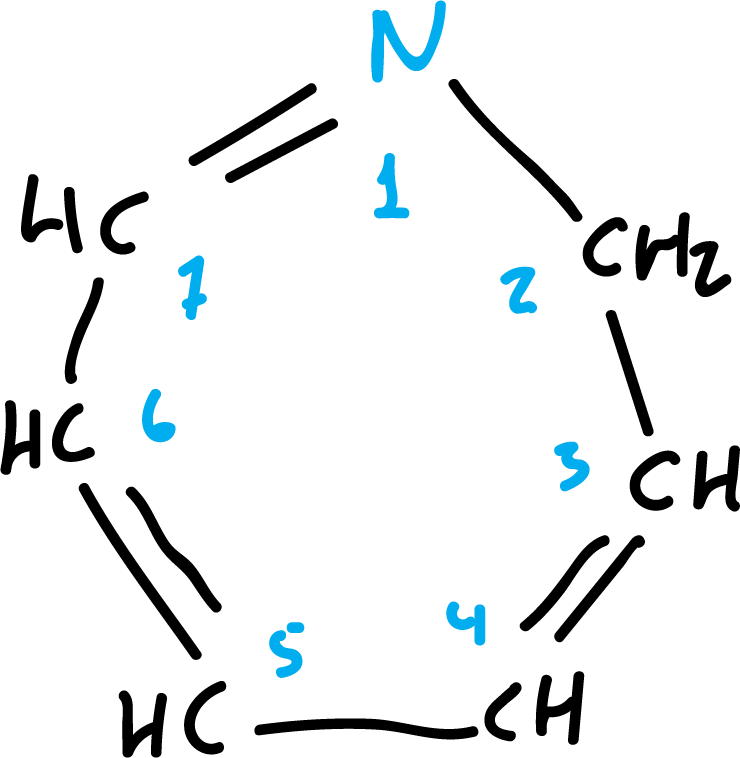
The most important systems are the 5- and 6-membered rings:
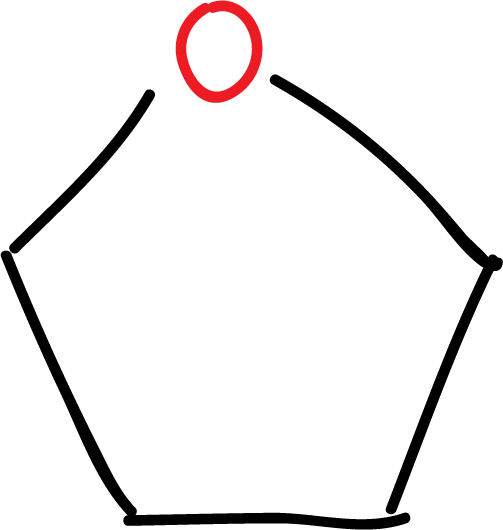
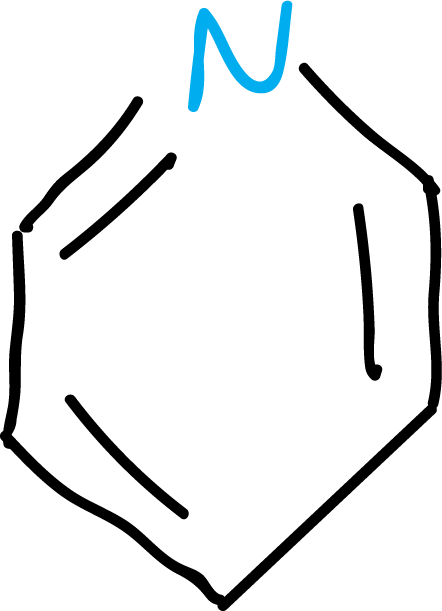
Within the heterocyclic systems, there can be saturated rings, for example tetrahydrofuran (THF), or unsaturated rings with at least one double bond. They can also be classified into non-aromatic and aromatic heterocycles, since there are heterocyclic systems that comply with the rule of aromaticity.
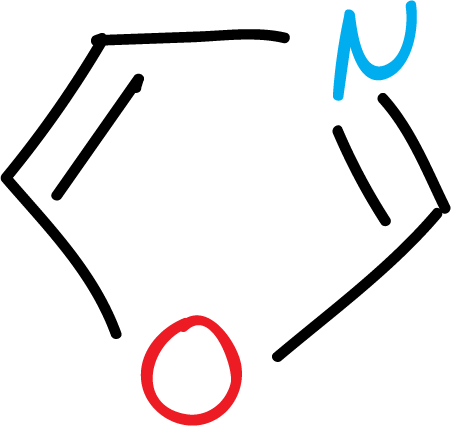
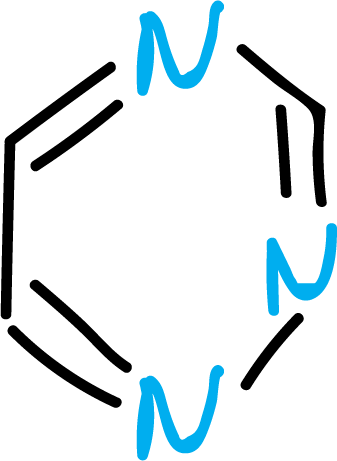
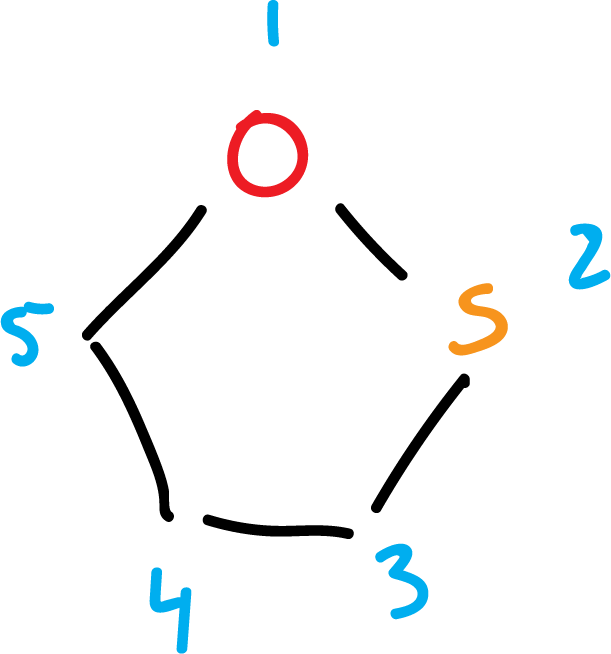
Another group of heterocycles corresponds to those with more than one heteroatom in the ring:

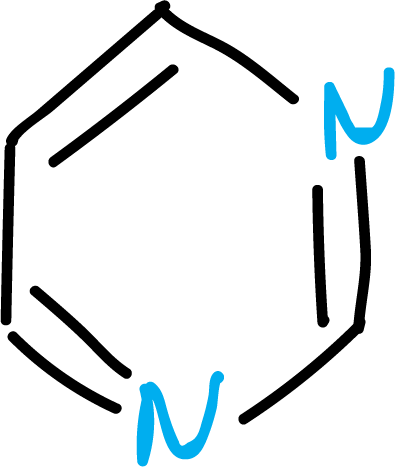
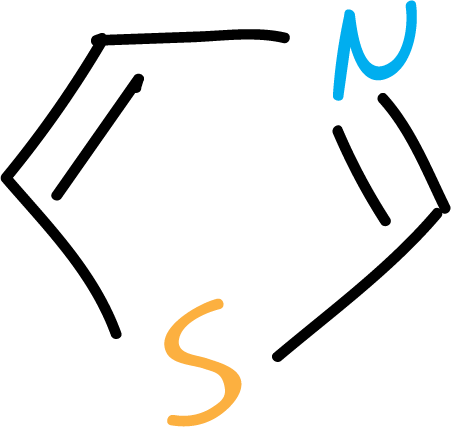
Also, there may be heterocyclic systems condensed to the benzene ring:
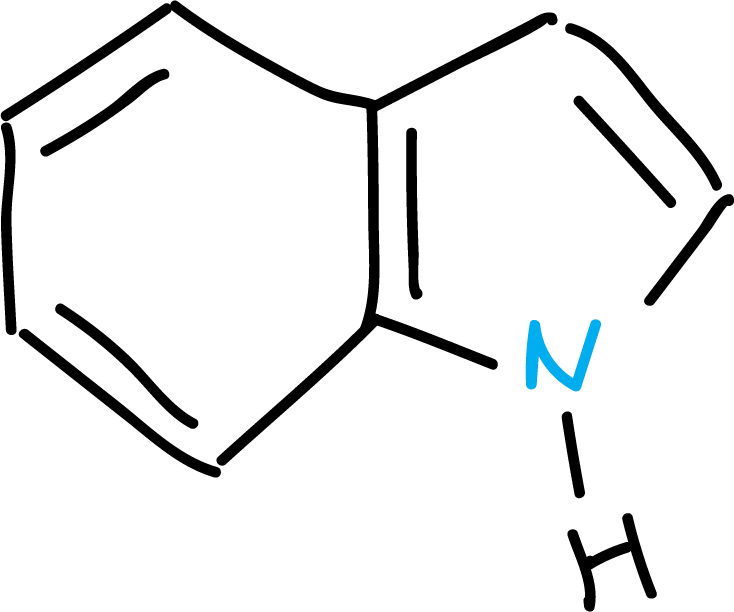
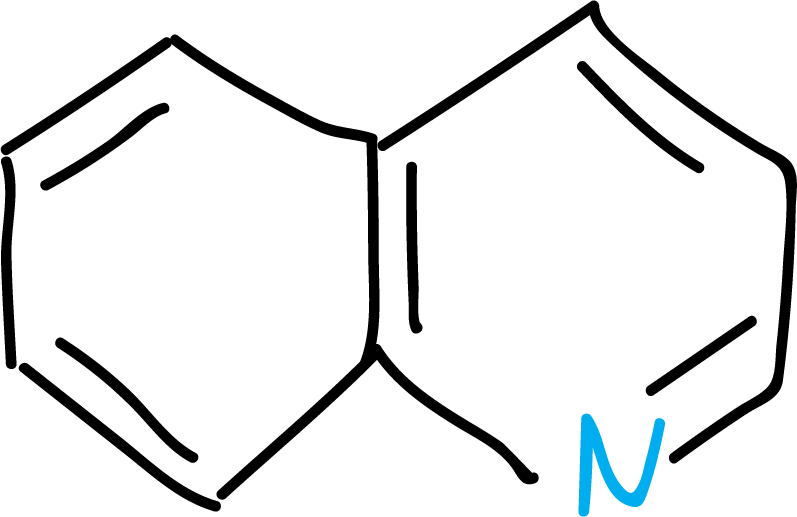
Several nomenclature systems have been developed for heterocyclic compounds. They can be named by recognized trivial names, by systematic names and also other nomenclature based on the names of the analogous cyclic hydrocarbons.
In general, they are major compounds in many of the current research papers.
One of the reasons for the extreme use of these types of chemical compounds is because their functionality can be modified by manipulating their structure. For example, by changing one heteroatom for another, or by moving the location of the heteroatoms themselves within the ring.
In addition, various functional groups can be incorporated as substituents or as part of the ring itself. They are used as intermediates in organic synthesis. For example, a relatively stable ring system can undergo a series of synthetic steps and then be broken down to generate other functional groups.
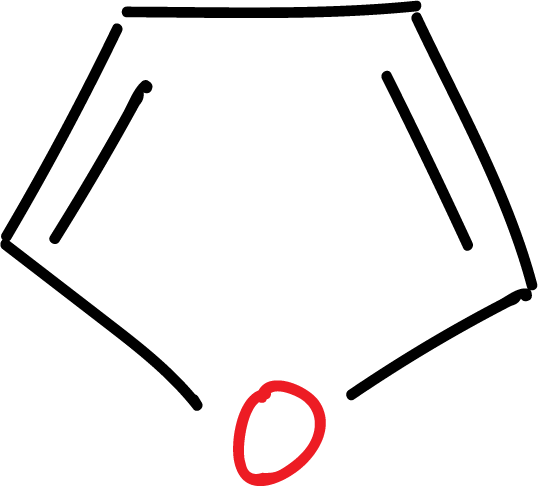
For example, furan derivatives can be employed as masked γ-dicarbonyl systems:
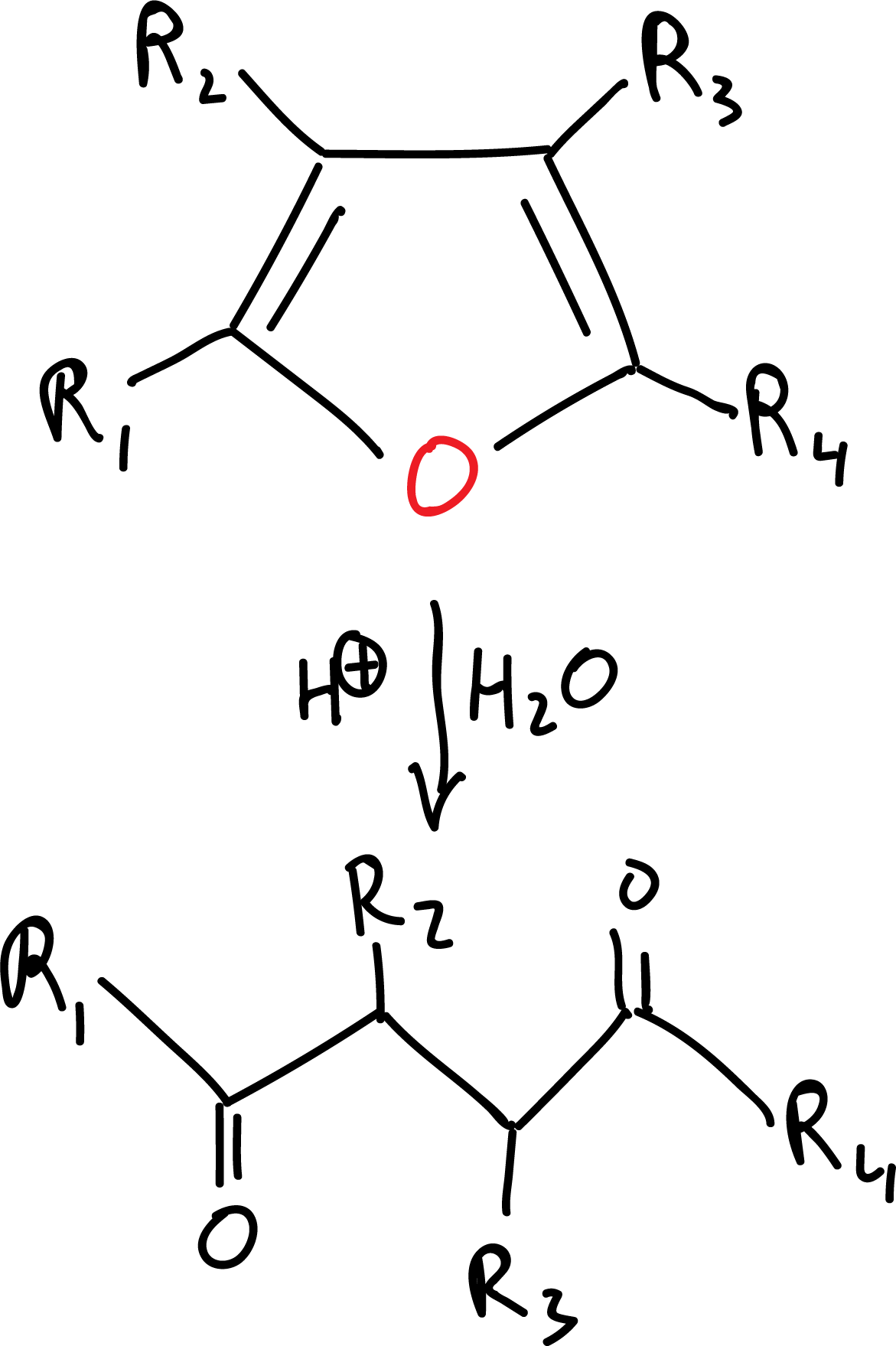
The ring can be broken with an acid to obtain the functional group.
Nomenclature
There are different nomenclature systems for heterocyclic compounds. Recognized trivial names, systematic namesand another nomenclature system based on cyclic hydrocarbons analogous to the compound to be named can be used.
However, the most frequently used system, especially for aromatic heterocycles, is a hybrid between trivial and systematic names using standard prefixes and suffixes (Hantzsch-Widman nomenclature system).
Hantzsch-Widman nomenclature
In this system, the prefix indicates the type or types of heteroatoms that can form the heterocycle.
The suffix tells us the number of terms in the ring, whether it has nitrogen or not, as well as whether they are saturated or unsaturated.
| Element | Valence | Prefixto |
| Oxygen | II | Oxa- |
| Sulfur | II | Thia- |
| Selenium | II | Selena- |
| Tellurium | II | Tellura- |
| Nitrogen | III | Aza- |
| Phosphorus | III | Phospha- |
| Silicon | IV | Sila- |
| a The final “a” is deleted when the prefix is followed by a vowel. | ||
When two or more distinct heteroatoms are present, the prefixes are arranged in the order shown in Table 1.
Two or more heteroatoms of the same type are indicated as di, tri, etc.
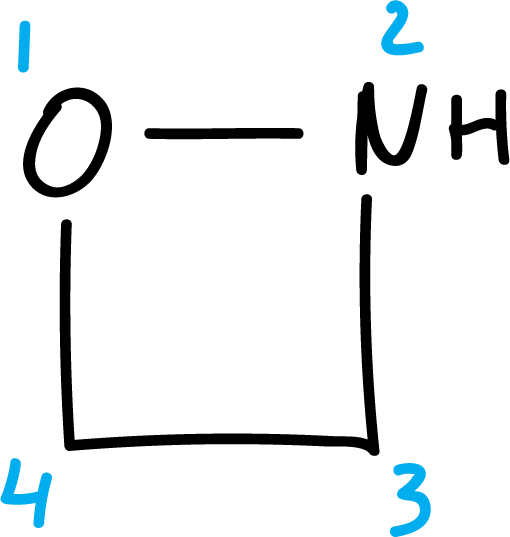
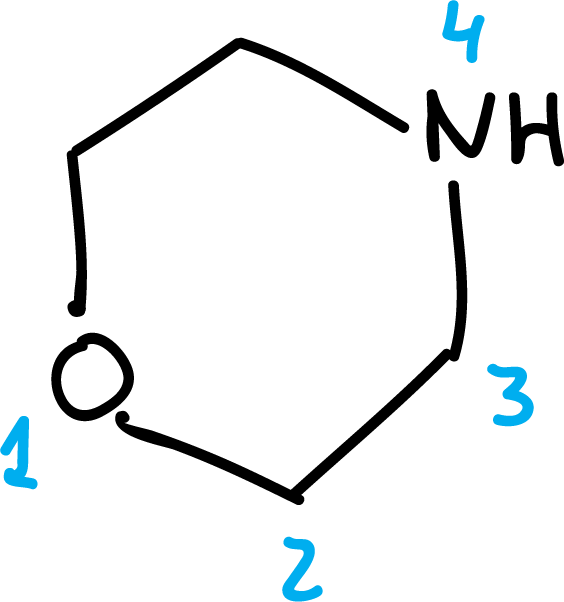
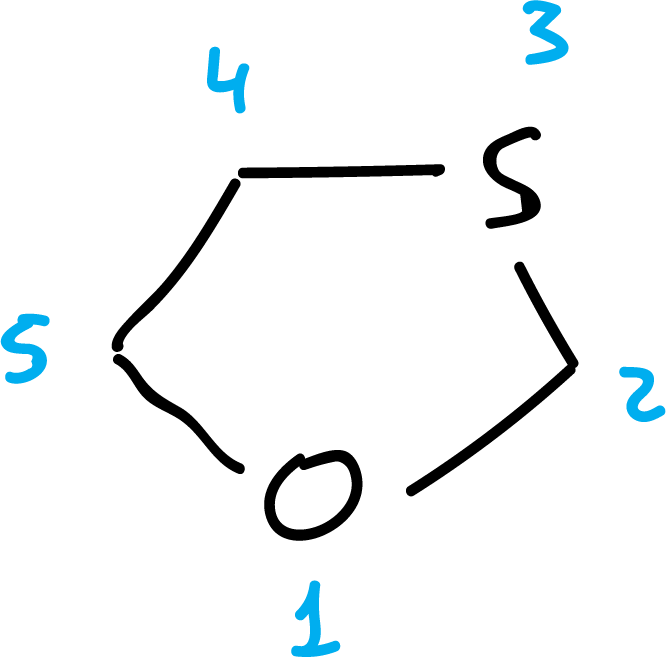
To number them, start at the heteroatom that appears higher in order Table 1.
Some examples of numbering are shown below:
| Ring size | Unsaturateda | Saturated |
| 3 | -irine | -iridine |
| 4 | -ete | -etidine |
| 5 | -ole | -olidine |
| 6 | -ine | b |
| 7 | -epine | b |
| 8 | -ocine | b |
| 9 | -onine | b |
| 10 | -ecine | b |
| a With the maximum number of non-cumulative double bonds (with the valences of Table 1). b It is expressed with the prefix -perhydro attached to the name of the unsaturated compound. | ||
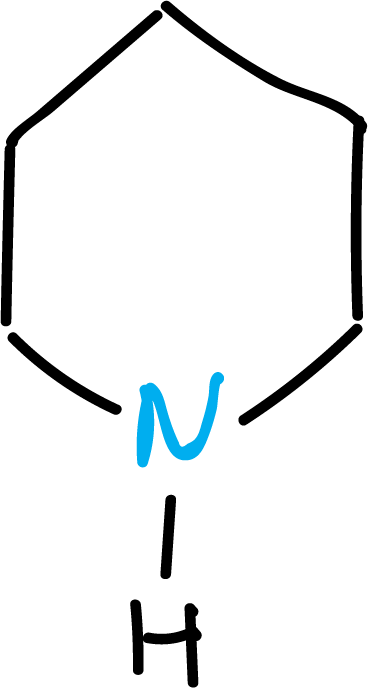
An example of the use of the prefix -perhydro would be:
| Ring size | Unsaturateda | Saturated |
| 3 | -irene | -irane |
| 4 | -ete | -etane |
| 5 | -ole | -olane |
| 6 | -ine | -ane |
| 7 | -epine | -epane |
| 8 | -ocine | -ocane |
| 9 | -onine | -onane |
| 10 | -ecine | -ecane |
| a With the maximum number of non-cumulative double bonds (with the valences of Table 1). | ||
Examples
Some examples of Hantzsch-Widman nomenclature are shown below:
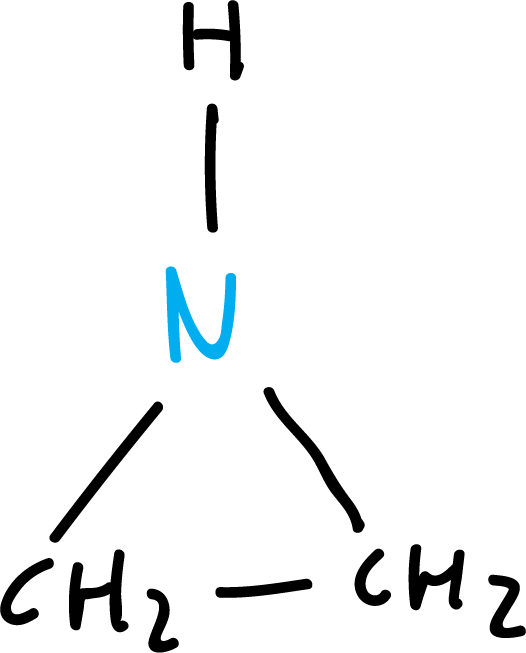
Heterocyclic systems that are not saturated, but to a lesser degree than that corresponding to the maximum number of non-cumulative double bonds, are named using the prefixes dihydro, tetrahydro, etc.
In 4- and 5-membered rings, a special termination is used for structures with one double bond, assuming that more than one non-cumulative double bond remains.
| Ring size | Rings with N | Rings without N |
| 4 | -etine | -etane |
| 5 | -ole | -olane |
Some examples are listed below:
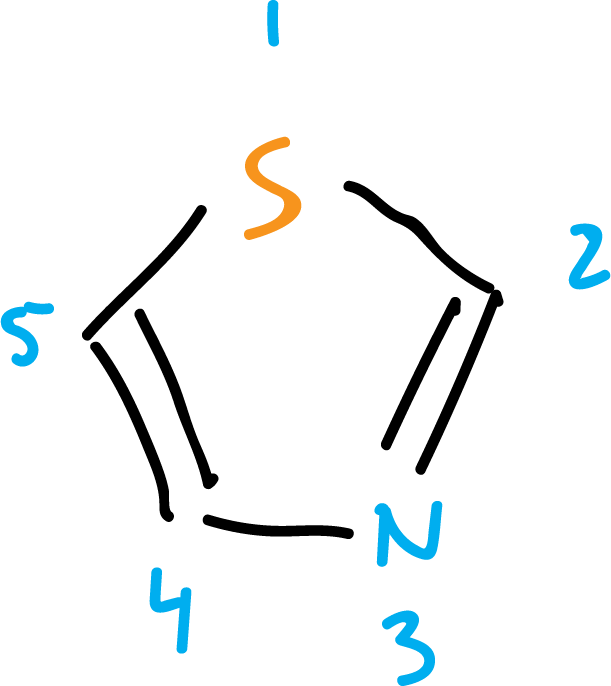
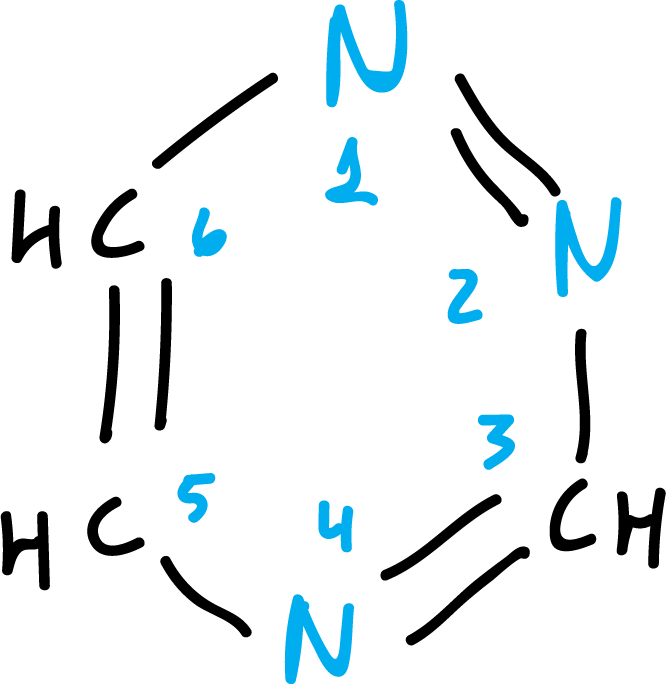
When the same heteroatom occurs more than once in a ring, the numbering is done in such a way that the locators are as low as possible, for example:
Trivial names of common ring systems
In the origins of organic chemistry, compounds were given names to identify them, almost always before their structure was known.
This is still done today for newly discovered natural products, the names of the compounds are usually based on the source of the compound or one of its most characteristic properties.
For example to the following aromatic heterocycle:
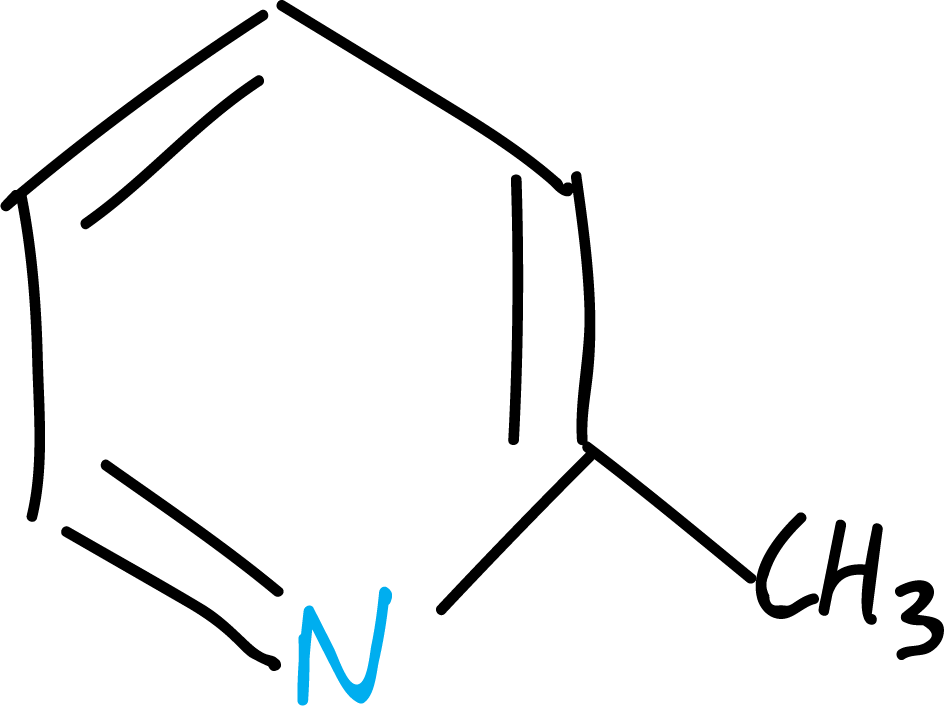
was named pyridine, based on the Latin word picatus, meaning tarry, because it was isolated from coal tar.
Pyrrole comes from the Greek word meaning fiery red, due to the heat produced by the compound with a pine splinter dipped in hydrochloric acid.
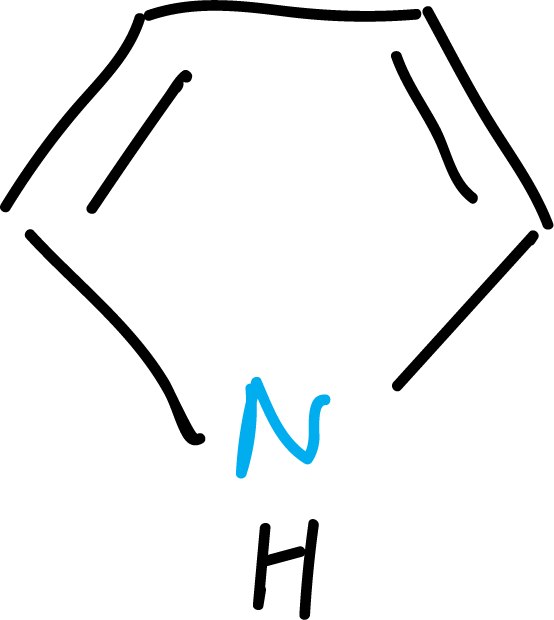
These trivial names contain no information about the structure of the compound and their use is being abandoned.
At present, approximately 60 trivial names survive and are recognized by IUPAC. These surviving names are important because they are used as a basis for constructing others.
Nomenclature of fused ring systems
Some heterocycles contain two or more fused rings, and many of them have trivial names, such as:
fig14a
Most of the fused heterocycles do not have a recognized trivial name, so the systematic name must be used. For this purpose, the common atoms are considered as belonging to both ring systems. The corresponding name is constructed by changing the names of the individual rings.
fig15a
These names are constructed following these steps:
1) The names of the components of the merged systems are chosen, whenever possible, from the list of recognized trivial names. The major component with a recognized name is selected. For example indole is better than pyrrole if the indolefragment is present. When the fragment has no recognized name, the systematic name is used.
2) In a fused system consisting of two or more individually named components, one of them is chosen as the base component for the compound name. e.g., pyrimidine is the base component in the example in the figure above (pyrrolo[1,2-a]pyrimidine).
3) The second component is added as a prefix to the name of the base component. This prefix is deduced by replacing the final “a” of the name of the ring system by “o”. For example, pyrazine -> pyrazine. There are some exceptions which are indicated in Table 5.
| Heterociclo | Nombre como prefijo |
| Furan | furo |
| Imidazole | imidazo |
| Isoquinoline | isoquino |
| Pyridine | pyrido |
| Quinoline | quino |
| Thiophene | thieno |
4) The links forming the ring system of the base component are numbered as a, b, c, … etc., starting with the side normally numbered 1,2.
The atoms forming the ring system of the second component are numbered in the normal way. The bonding bond is indicated by the appropriate letter and number, the numbers of the second component being mentioned in the sequence in which the base component occurs. For example:
fig16a