Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Aldehydes and Ketones – solutions to problems.
Solution 1:
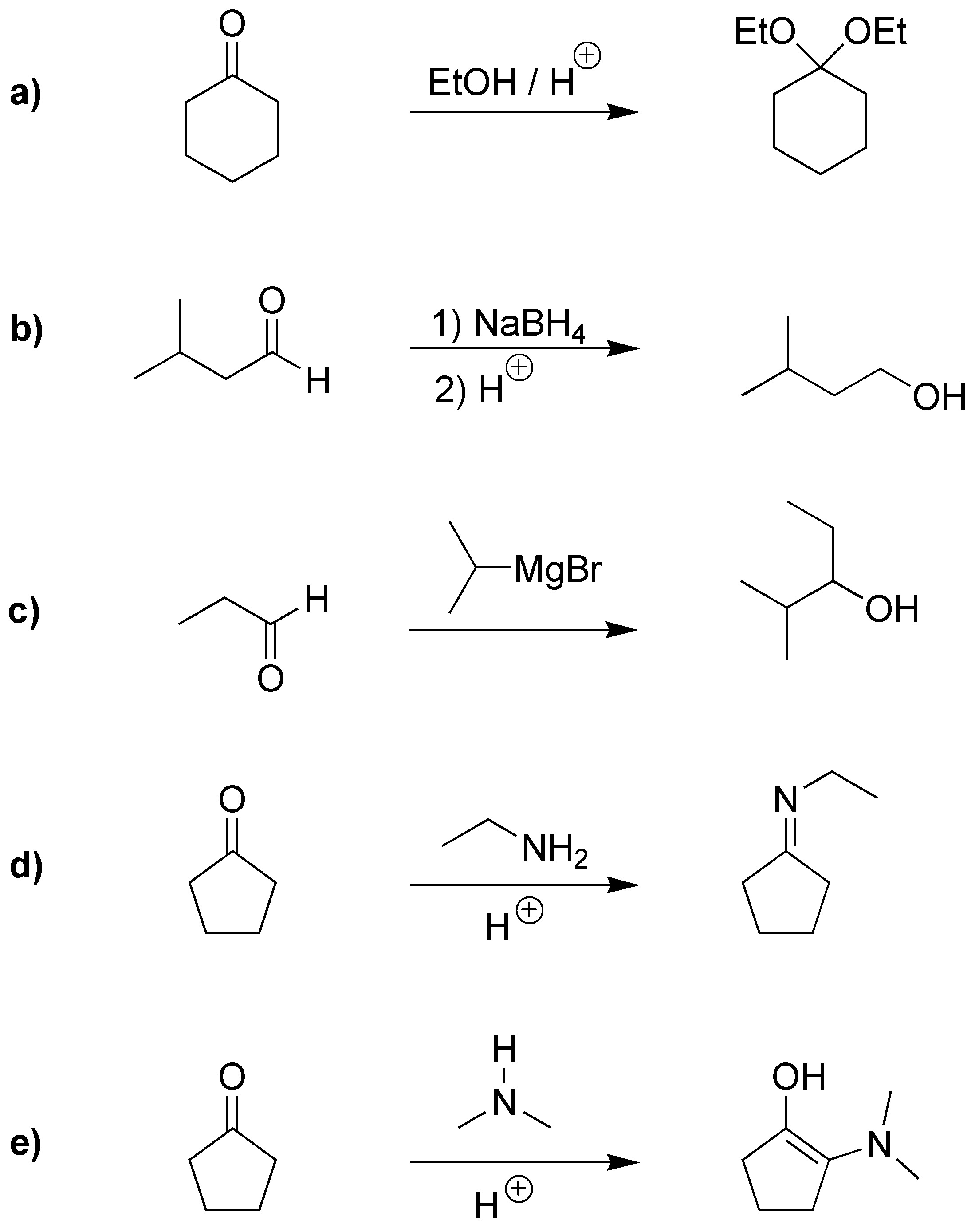
a) Hydration of an aldehyde or ketone results in the formation of the corresponding hydrate. b) Reduction produces the corresponding alcohol. c) Grignard compounds add nucleophilically to aldehydes producing secondary alcohols. d) Primary amines add to ketones producing imines. e) If the amine is secondary enamines are produced.
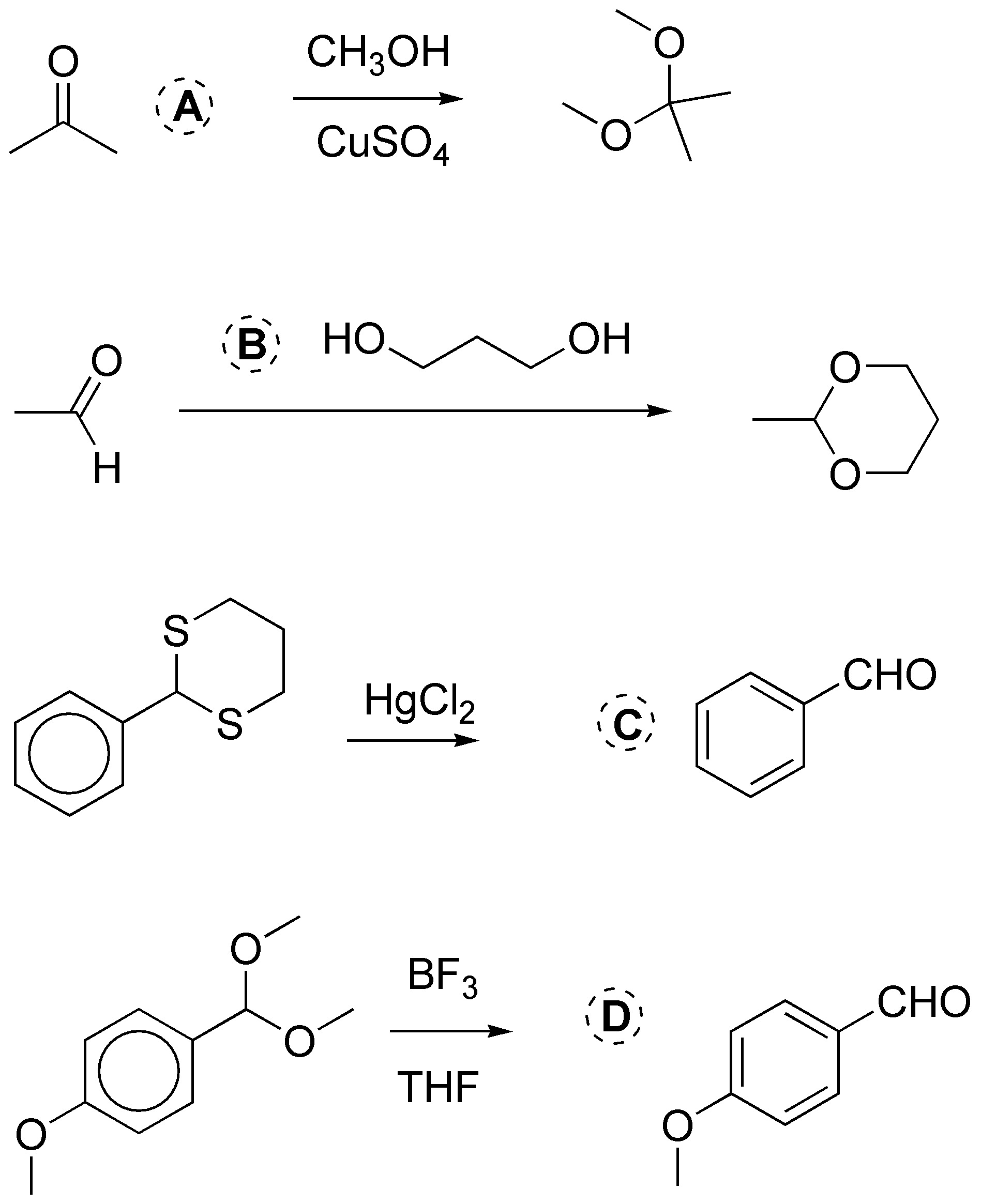
Solution 2:
a) Aldehydes when treated with alcohols in acidic medium are transformed into the corresponding hemiacetals. If the reaction continues, they are transformed into the corresponding acetal. b) Hydroxy aldehydes are usually in equilibrium with their hemiacetal form. c) HCN is added to aldehydes and ketones forming the corresponding cyanhydrin.
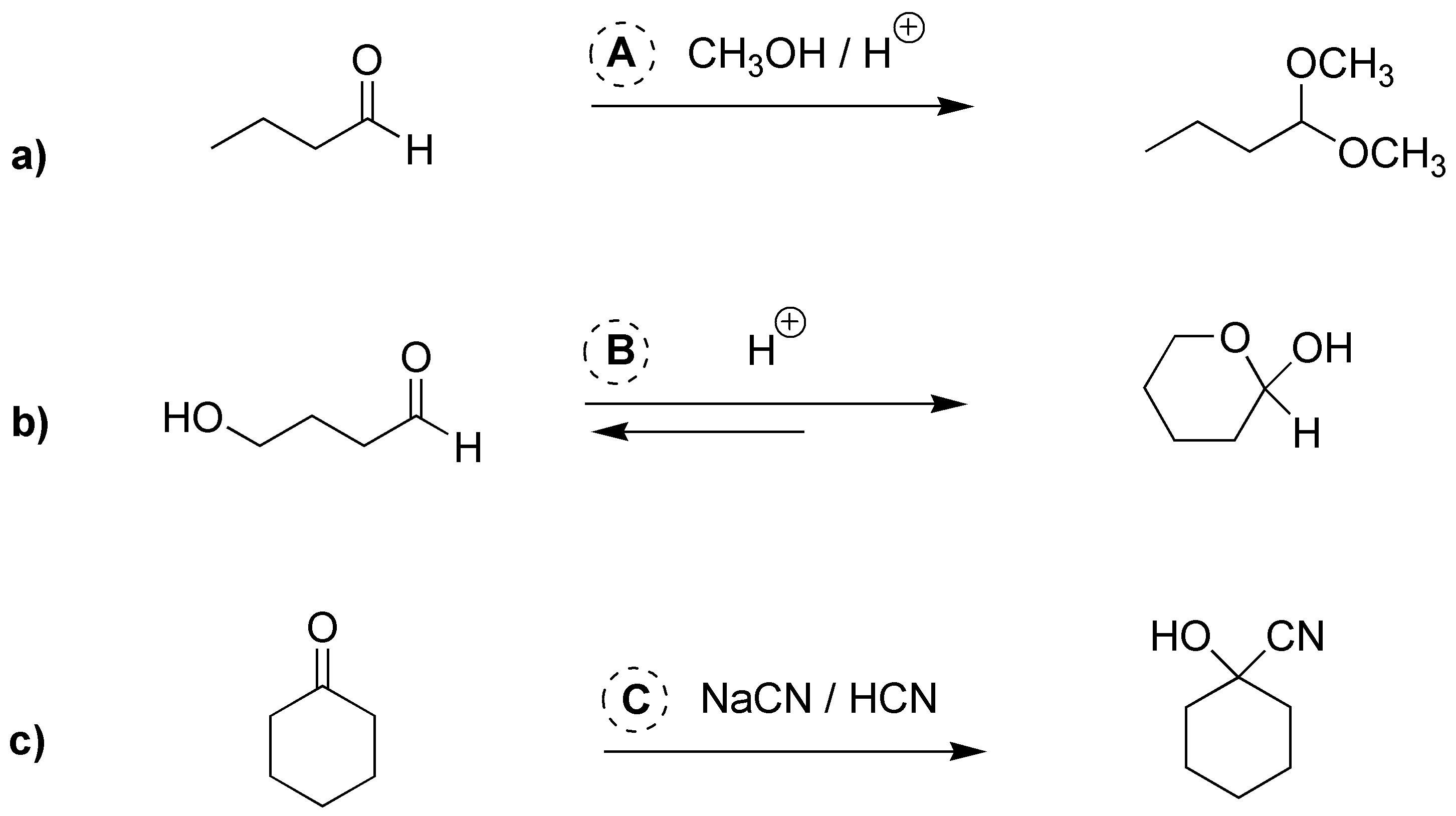
Solution 3:
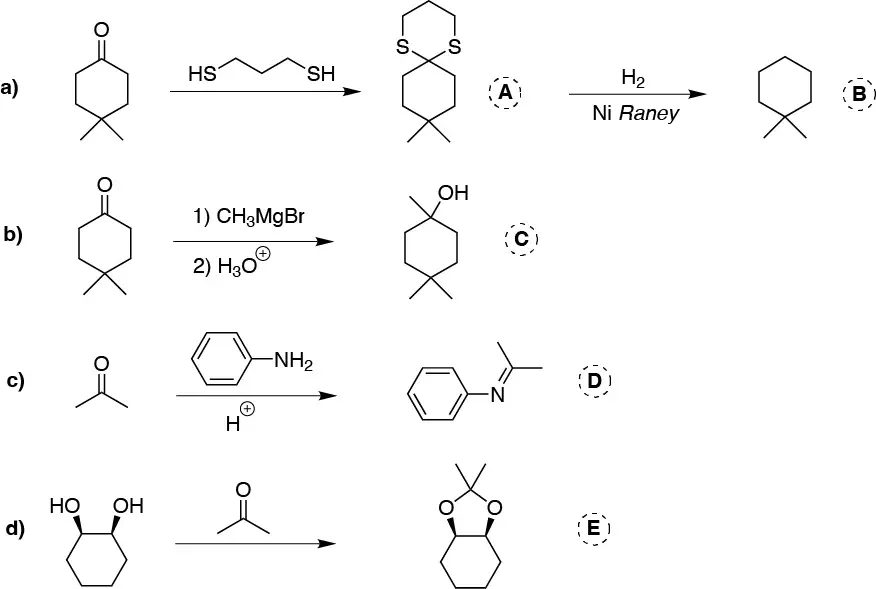
a) The aldehydes and ketones react with thiols to form the corresponding thioacetals which by catalytic hydrogenation produce the corresponding alkane. Giving as an overall result of both reactions deoxygenation (reduction) to alkane. b) Magnesians (Grignard compounds) add to carbonyls giving alcohols. c) Primary amines add to carbonyls producing imines. d) Glycols react with aldehydes and ketones producing cyclic ketals and acetals.
Solution 4:
a) As the product is a cyanhydrin resulting from the addition of HCN to cyclopentanone. b) Phenylhydrazine reacts with ketones giving hydrazones, then B must be 3-pentanone. c) The final product is an acetal so it must result from the addition of two alcohols to a carbonyl compound, 1,9-dihydroxy-5-nonanone. d) Like Grignard compounds, organolithiums react with aldehydes to produce secondary alcohols, in this case it will be cyclohexylmethanal (cyclohexylcarbaldehyde).
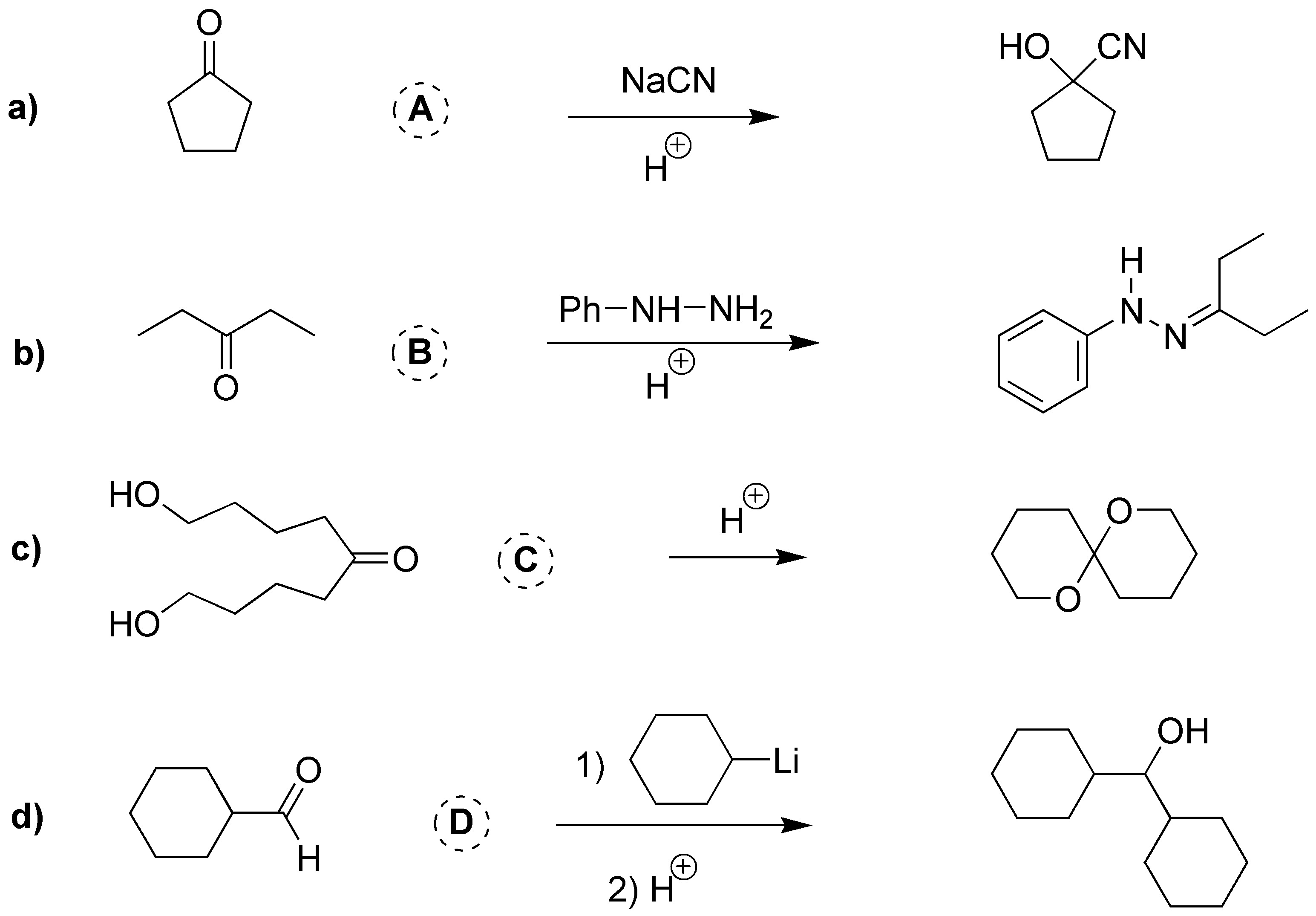
Solution 5:
a) To avoid mixtures of ketone oxidation products, oxidation with strong oxidants is used with symmetric ketones including cyclic ketones. Cyclic ketones, such as cyclohexanone, are oxidized to the corresponding dicarboxylic acid.
b) and c) Aldehydes such as benzaldehyde, or furfural, are oxidized to the corresponding acids with mild oxidizing agents, Ag2O, or strong oxidizing agents, KMnO4.
d) However, conversely, ketones do not react with mild oxidizing agents, Ag(NH3)2+.
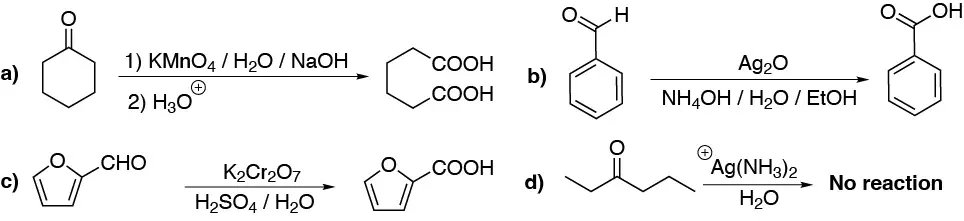
Solution 6:
This is a Baeyer-Villiger oxidation. The intermediate formed is described in the figure. In principle, both methyl, a, and phenyl, b, could migrate. Because the order of priority is higher for phenyl the major product is phenyl acetate.
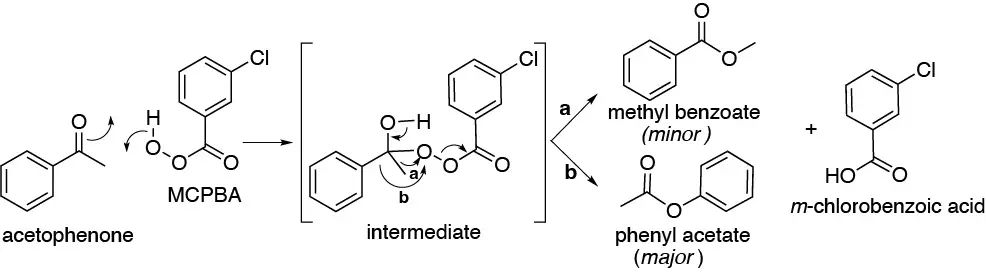
Solution 7:

Catalytic hydrogenation transforms aldehydes and ketones into alcohols. Consider that the C=C double bond is more reactive than the C=O carbonyl, so both will be reduced if they are present in the same molecule (compounds I and II). In addition, if the ketone has two different substituents (I), a chiral carbon is generated giving rise to a racemic (mixture of the two enantiomers R and S).
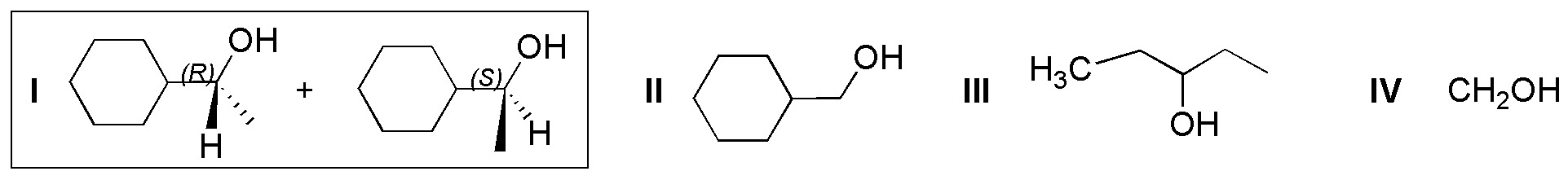
Solution 8:
LiAlH4 is used in conjunction with aprotic solvents (ethyl ether, THF, etc.) because it is very reactive (reacts explosively with water and alcohols, releasing hydrogen which can cause fires). LiAlH4 readily reduces aldehydes and ketones, but has the disadvantage that it can also reduce esters and carboxylic acids (see Table A6 of reductants and oxidizers).
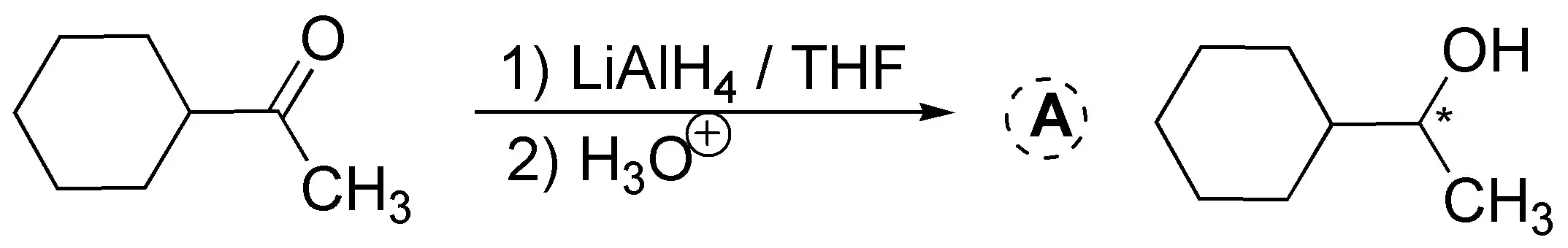
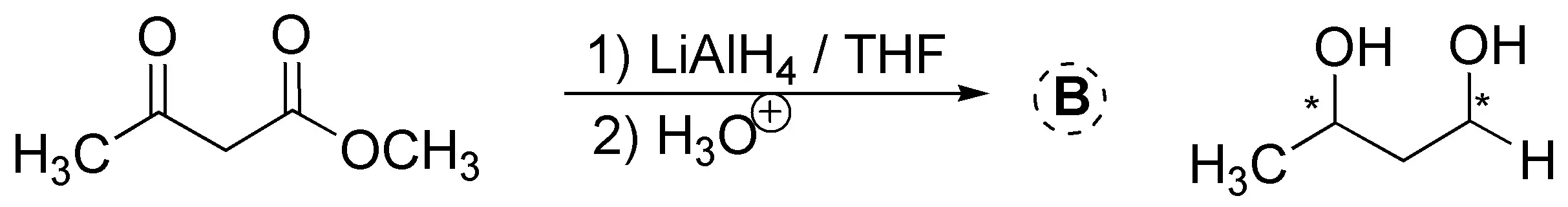
Solution 9:
NaBH4 unlike LiAlH4 is a milder and more selective reductant, it reacts slowly with alcohols and with water (at basic pH) proof of this is that alcohols are used as solvents (EtOH, MeOH, etc.) in these reactions. It only reduces aldehydes and ketones, without interfering with other carbonyl groups (esters and carboxylic acids) present in the molecule. In both cases, a racemic mixture is obtained, since a chiral center is generated in the reduction.
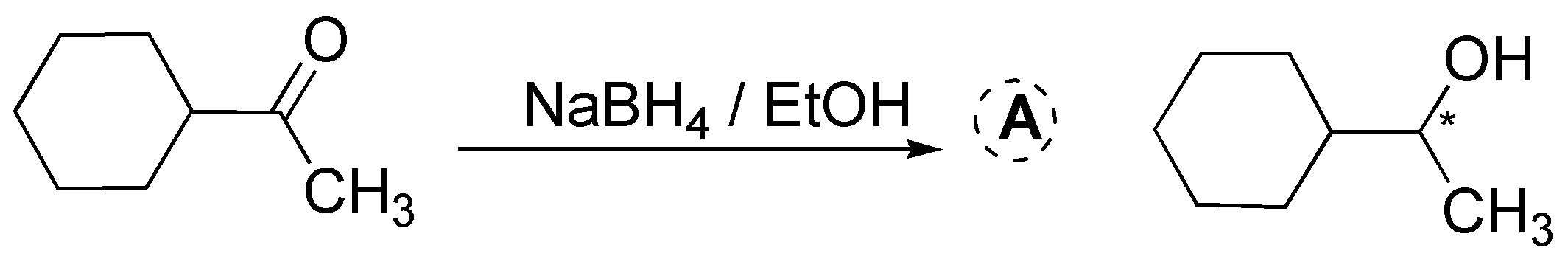
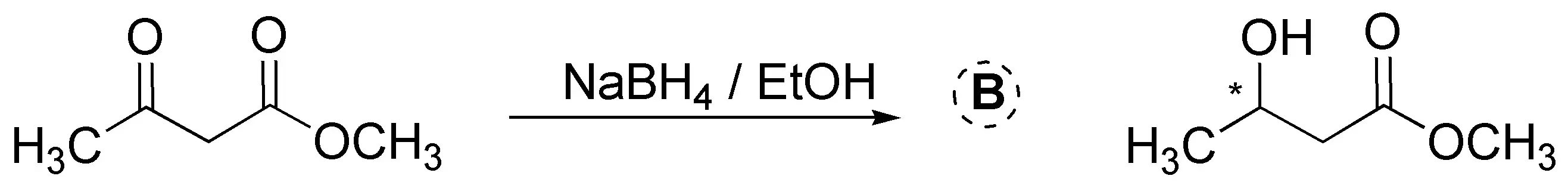
Solution 10:
There are two possibilities for the formation of 2-methylpent-2-ene by the Wittig reaction, the first starting from acetone and the second option from propanaldehyde, these must react with the corresponding ilides shown in the figure.
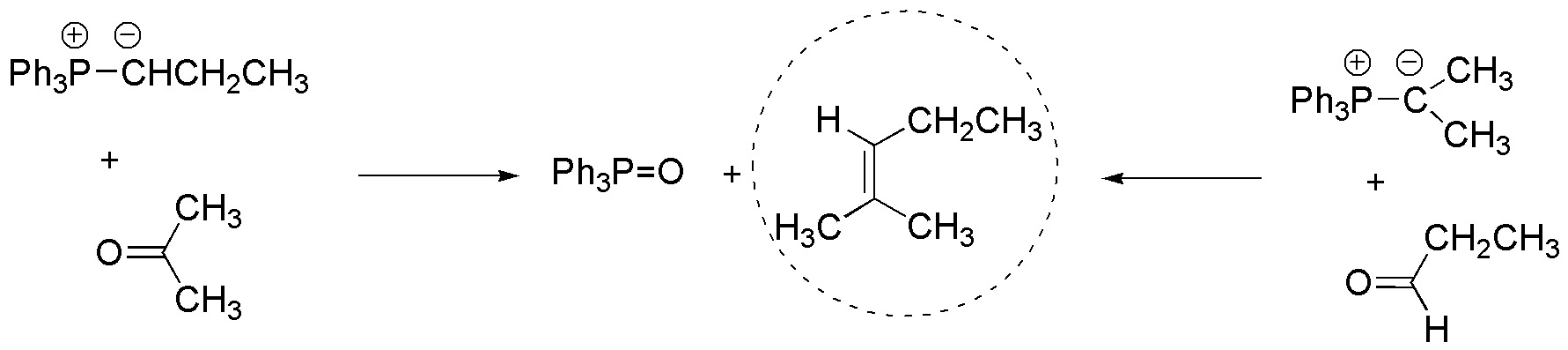
Solution 11:
In both cases, the first step of the reaction corresponds to an addition to the carbonyl carbons of the ethyl, a), and propinyl, b), groups, respectively; forming the corresponding alkoxides. Subsequently, a protonation step of said alkoxide occurs to give the tertiary alcohols of the two reactions a) and b).

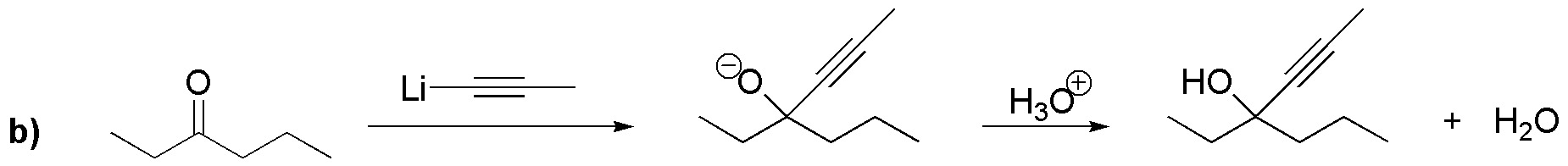
Solution 12:
The pH is crucial in this type of reactions. If the reaction medium is too acidic, which is necessary for the carbonyl to be protonated increasing reactivity, the amine is also protonated and the nucleophilic power (absence of the electron pair) disappears. We must choose compromise conditions, carrying out the reaction in a buffer solution with optimum pH of 4.5.
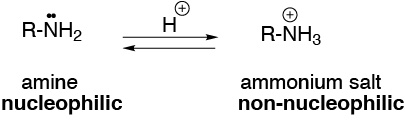
Solution 13:
Corresponds to a reductive amination, a process (in two steps) in which a ketone is transformed into an amine. First, the attack of the amine on the carbonyl and the loss of water occurs, an intermediate A, Schiff’s base (imine), is generated, which is subsequently reduced by hydrogenation to amine B.
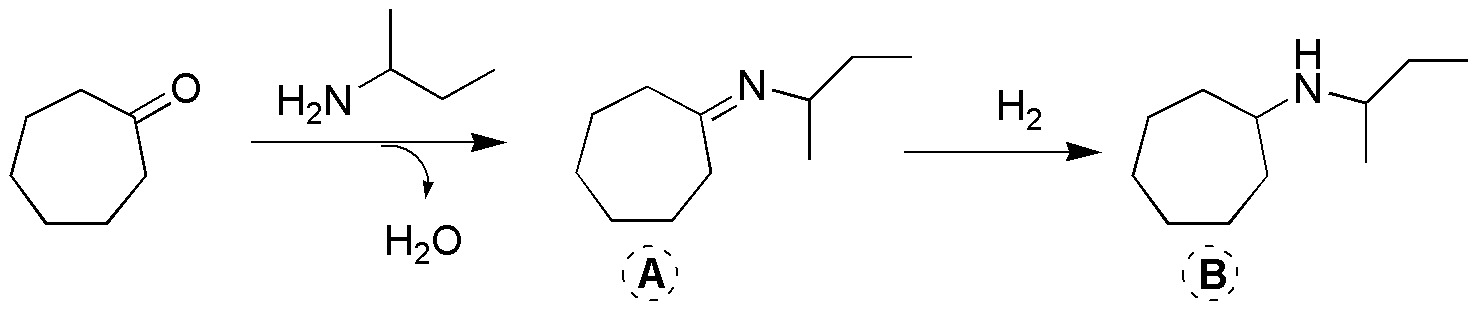
Solution 14:
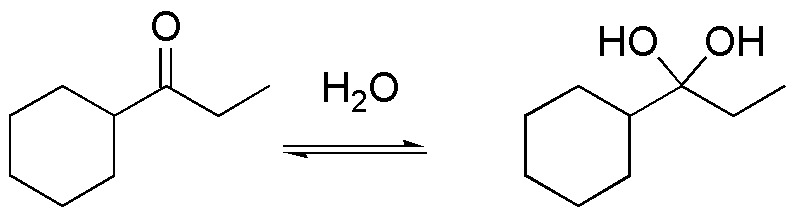
a) Compound I is a hydrate of a ketone. They can be obtained by treatment of the starting ketone with water. The reaction is an equilibrium process, where the value of the equilibrium constant is usually less than 1, except for formaldehyde which has a value of 18. The process is catalyzed in the presence of an acid and is of no synthetic interest.
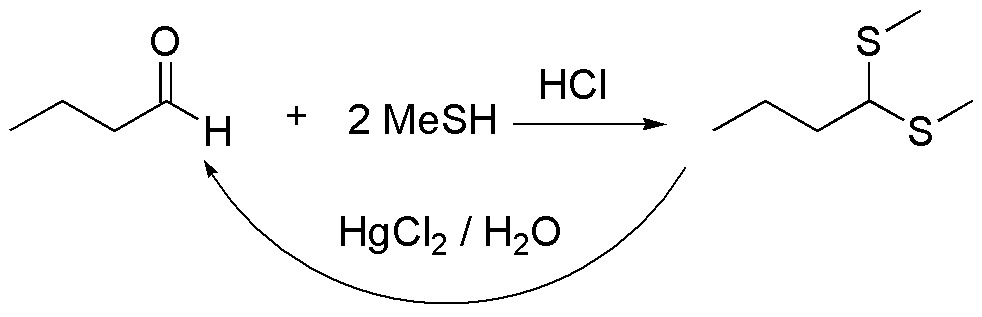
b) Compound II is a thioacetal, which comes from the reaction between butanal and methanethiol. The reaction is catalyzed by mineral acids, such as hydrochloric acid or Lewis acids. Thioacetals are stable in basic media, against nucleophiles and electrophiles, except MeI, so they are used as protecting groups of aldehydes and ketones, which can be regenerated by treating them with mercuric salts.
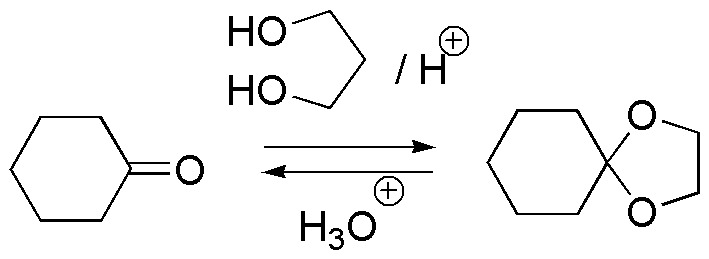
c) Compound III is a cyclic acetal that comes from a ketone, cyclohexanone, and an alcohol with two hydroxyl groups on the same molecule, generating a second five-membered cycle in which two of the component atoms of the cycle are oxygen atoms. Acetals are stable in basic media against nucleophiles, oxidizers and reductants. The carbonyl starting compound is regenerated by acid hydrolysis.
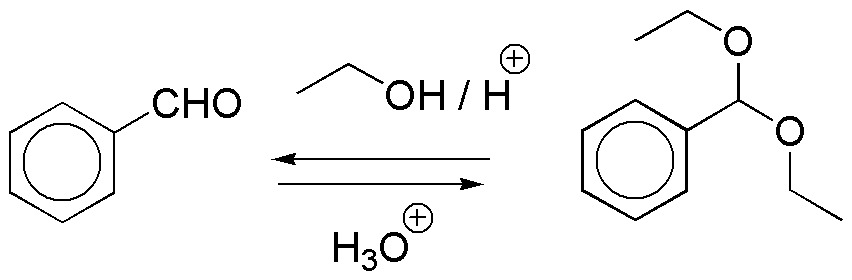
d) Again it is an acetal obtained from benzaldehyde and ethanol in acid medium. As in the previous case the starting compound is obtained by acid hydrolysis.
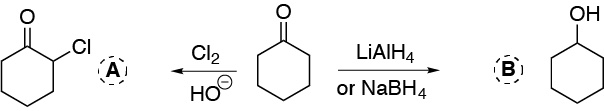
Solution 15:
a) The first reaction corresponds to the halogenation of a ketone. The hydrogens adjoining a carbonyl group are relatively acidic and in a basic medium can be replaced by a halogen (chlorine or bromine). b) The reduction of a ketone produces the corresponding secondary alcohol.
Solution 16:
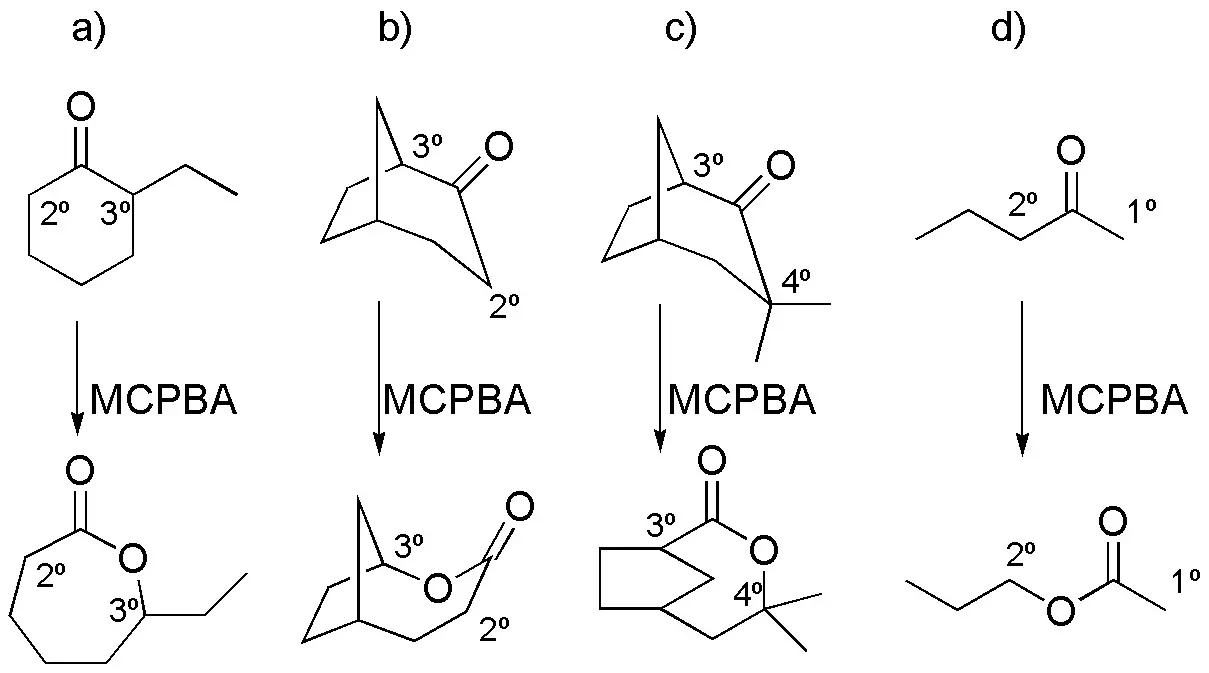
Of the two possible products of the Baeyer-Villiger oxidation reaction, the ester from the cleavage of the C-C bond corresponding to the most substituted carbon is mostly observed, due to the higher migratory fitness.
Solution 17:
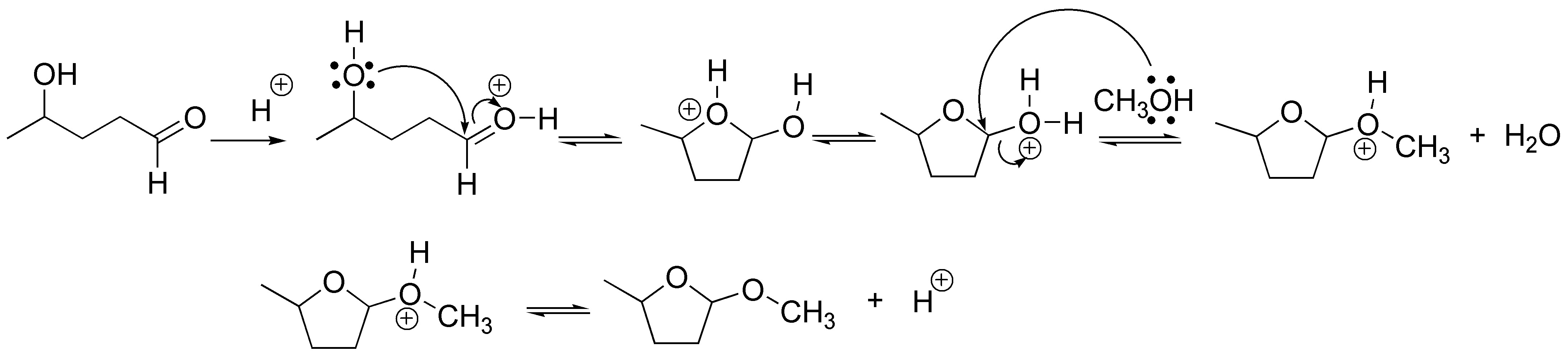
4-hydroxypentanal in acidic medium is forming a cyclic hemiacetal which upon protonation can be substituted by a nucleophile such as methanol producing the corresponding cyclic acetal.
Solution 18:
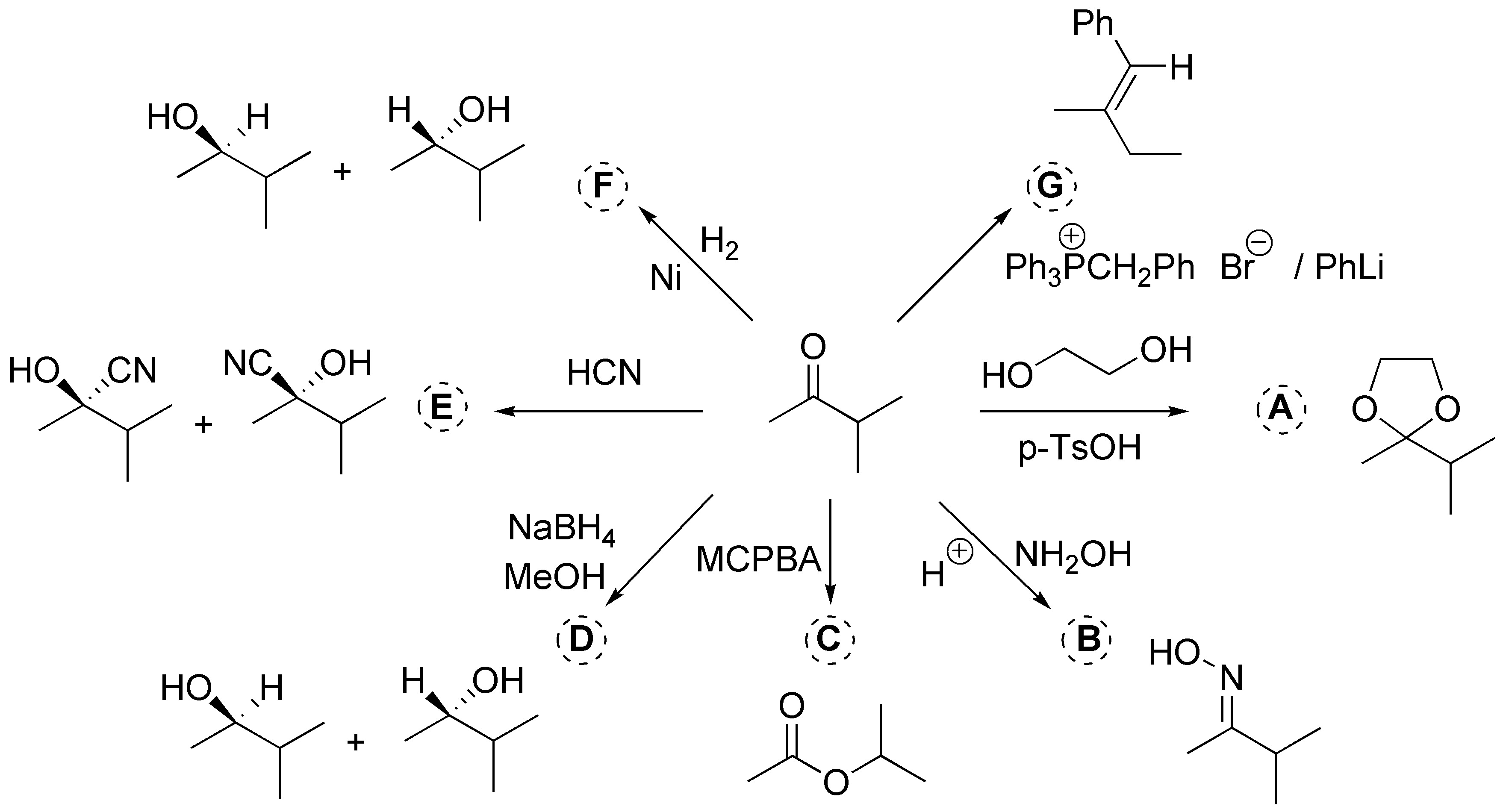
a) corresponds to the formation of a cyclic acetal; b) the formation of an oxime; c) a Baeyer-Villiger oxidation; d) a reduction; e) formation of a cyanhydrin; (f) hydrogenation (reduction); and g) is a Wittig reaction.
Solution 19:
a) It is the formation of an imine, as the amine is primary; b) formation of an enamine as the amine is secondary; c) hydration of an aldehyde (formation of a hydrate) and d) α-halogenation of a ketone:
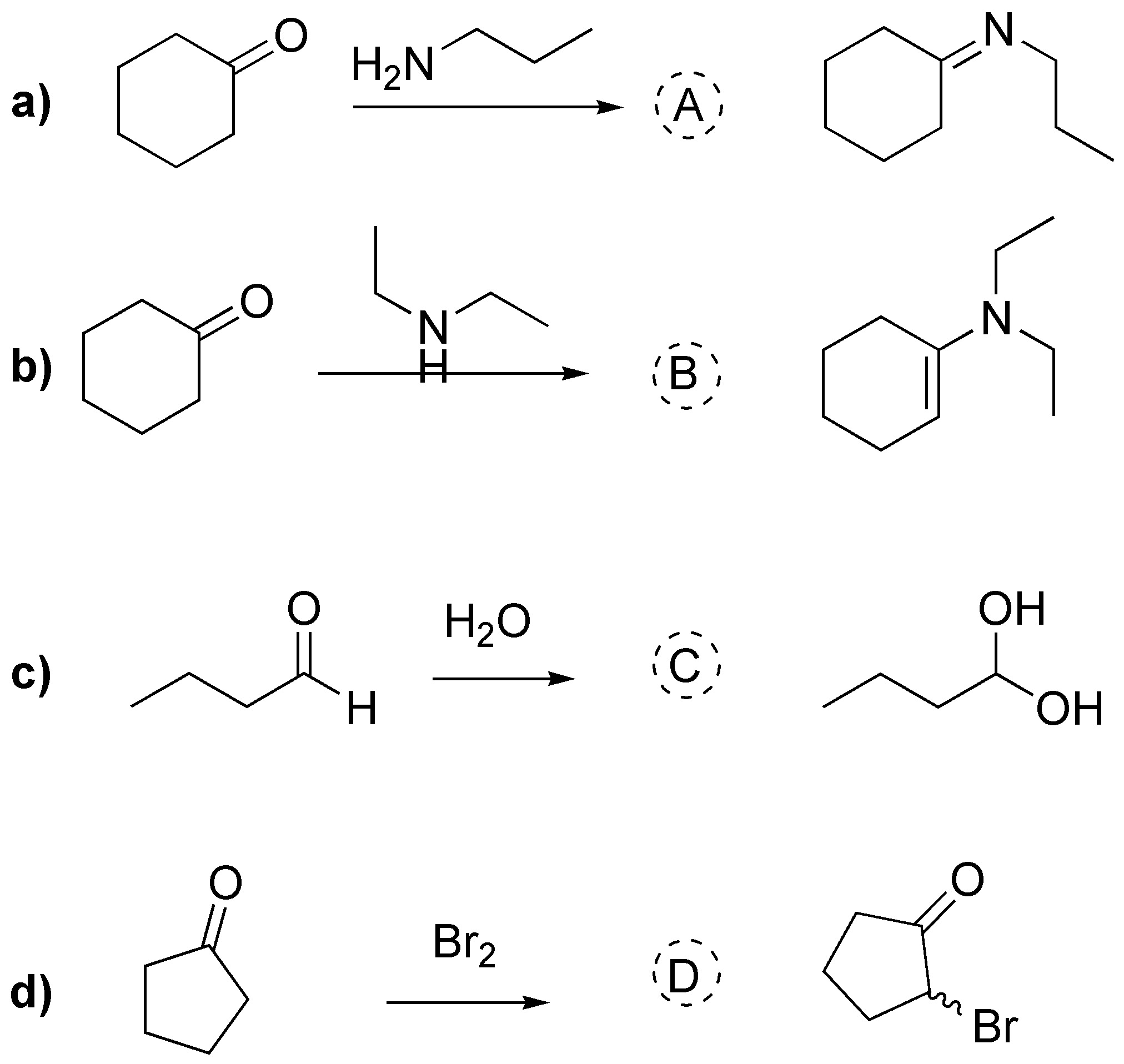
Solution 20:
The reactions necessary to transform cyclopentanone into these three products would be: A reduction to give a), the addition of an organometallic to give b) and a Wittig reaction to give the hydroxyalkene c).
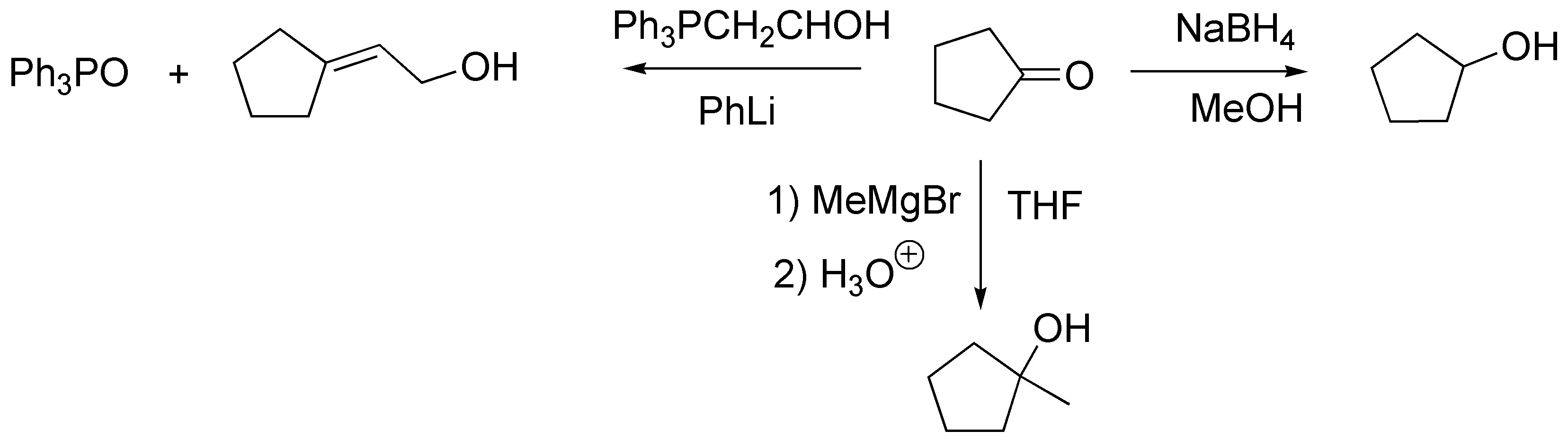
Solution 21:
The necessary condition to give the Cannizzaro reaction is that the group the carbon atom adjoining the aldehyde does not possess any hydrogen so A and C will give the Cannizzaro reaction.

Solution 22:
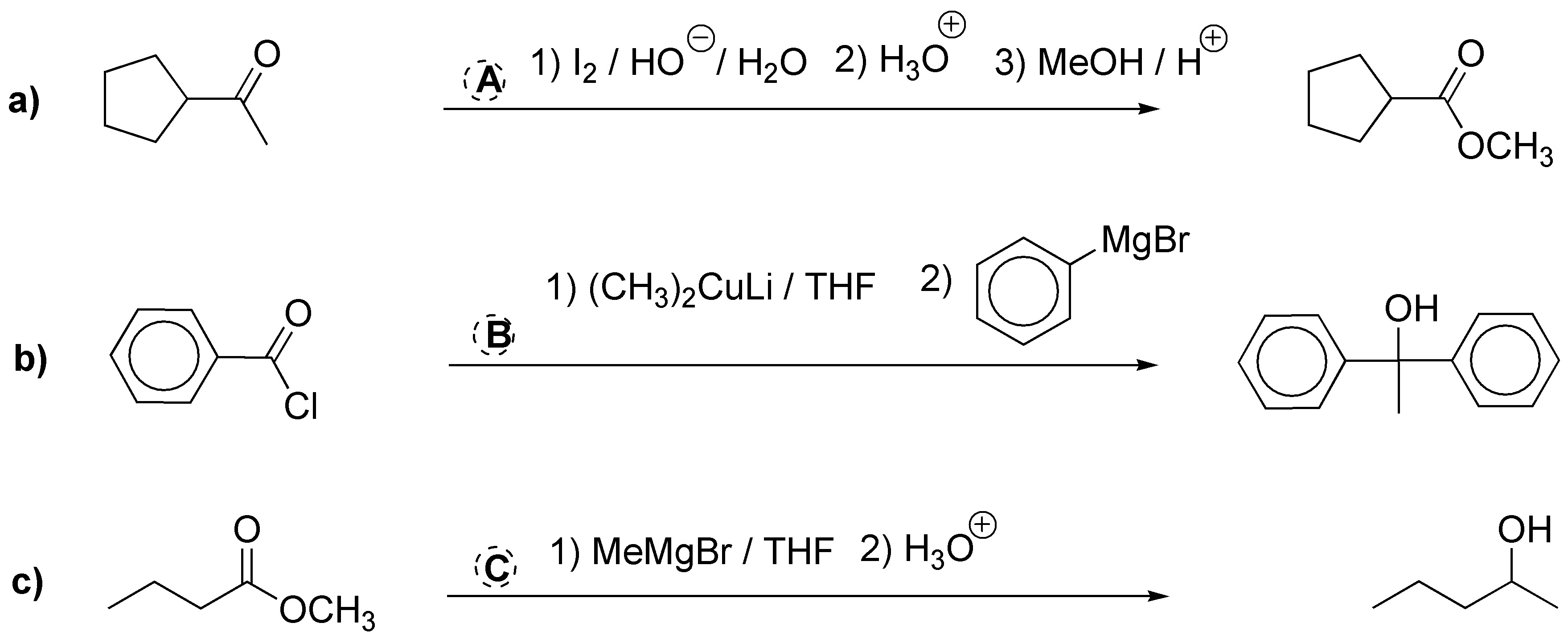
Solution 23:
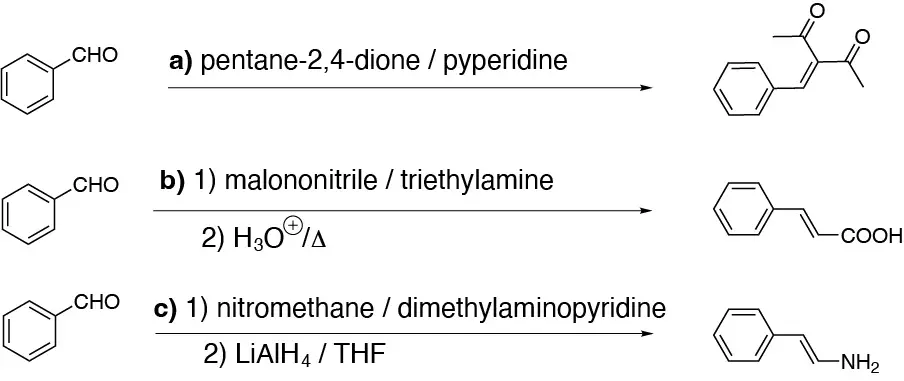
Solution 24: