Written by J.A Dobado | Last Updated on April 22, 2024
Go to the page with the list of problems.
Amines – solutions to problems
Solution 1:
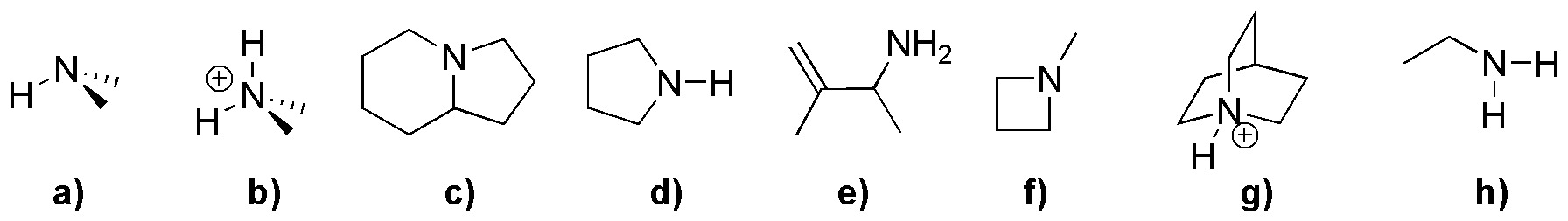
Nitrogen in primary amines presents valence 3 and is bound to two protons, therefore compound e) and h) correspond to this type of amines. In secondary amines also nitrogen has valence 3 but with only one hydrogen, as in the case of a) and d), note that, although compound g) has a hydrogen bonded to nitrogen, the valence is 4 and therefore it is not a secondary amine but a quaternary one. In compounds c) and f), the nitrogen has valency 3 and is not bonded to any hydrogen, therefore they are tertiary amines. Finally, when the nitrogen presents valence 4 (positive charge) it is called quaternary ammonium, independently of the hydrogens to which it is bound, this is the case of compounds b) and g).
Solution 2:
Gabriel synthesis consists of two sequences of reactions in which the overall process is the replacement of a halogen by an amino group. Therefore A will have the same structure as the starting bromide with the only difference that instead of Br there is NH2. The intermediate formed is a tertiary amine of the potassium phthalamide in which K is replaced by the same skeleton as the starting bromide. Therefore B corresponds to the structure shown in the figure below. As mentioned above, the starting alkyl halide and the amine obtained as a product share the same carbon skeleton, so C can be the product by exchanging NH2 for Br.
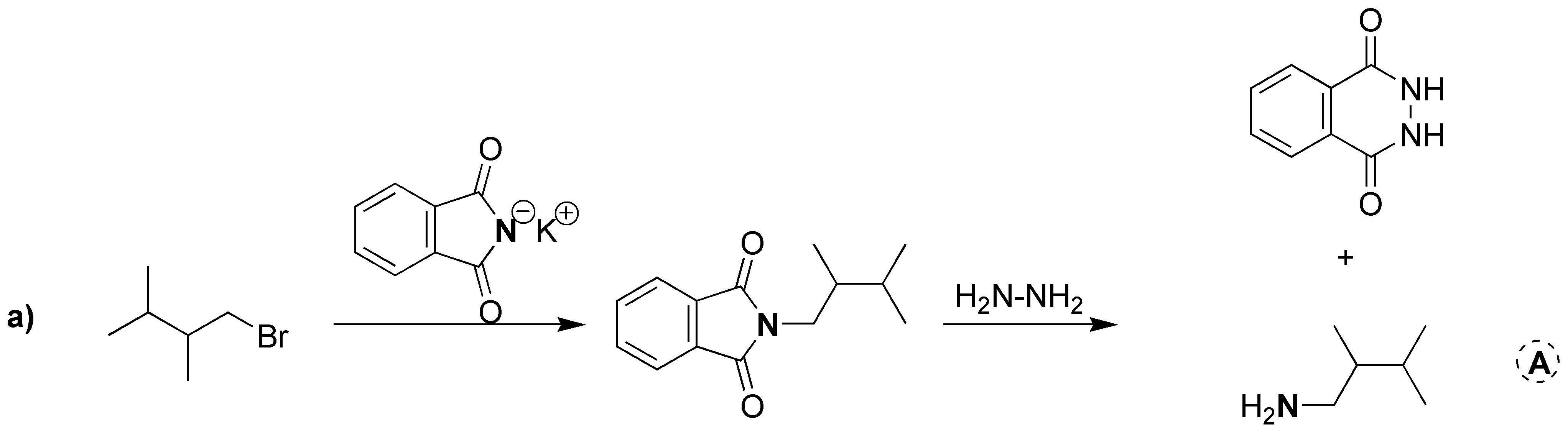
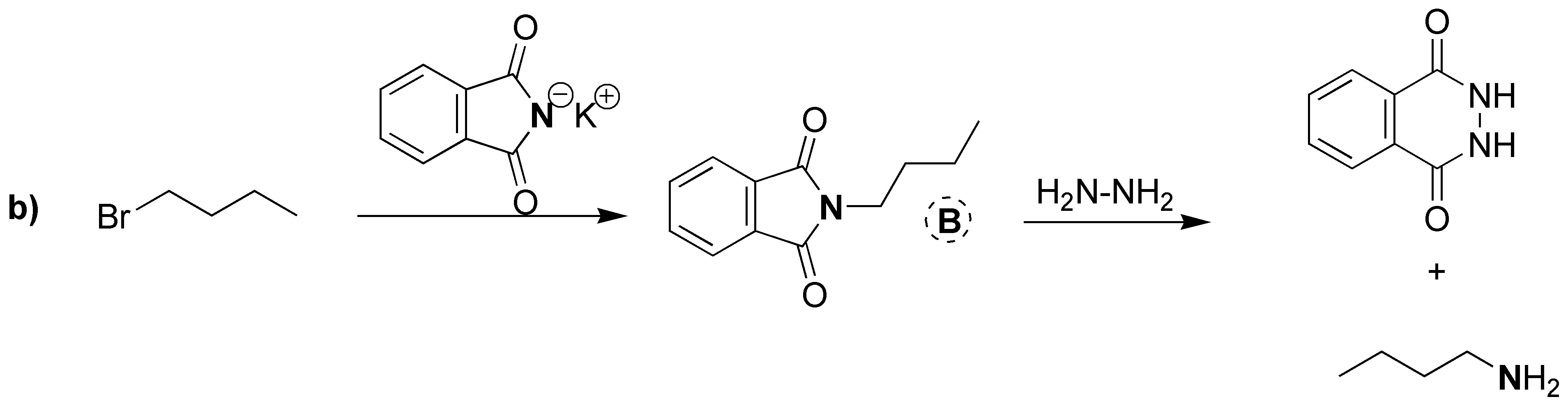
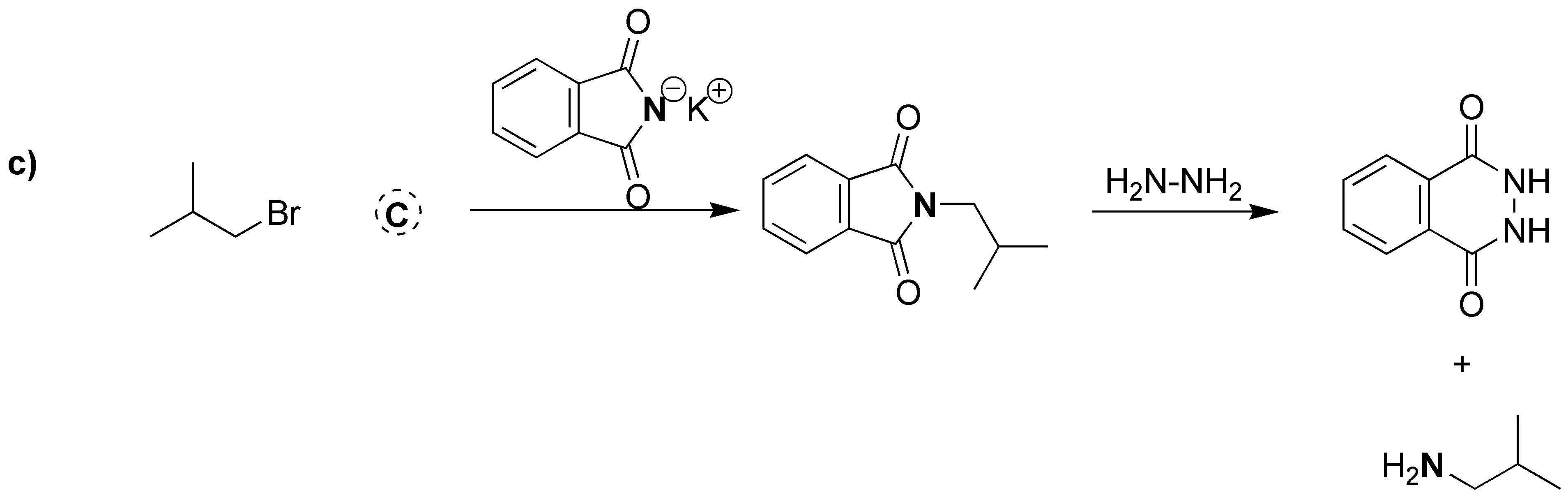
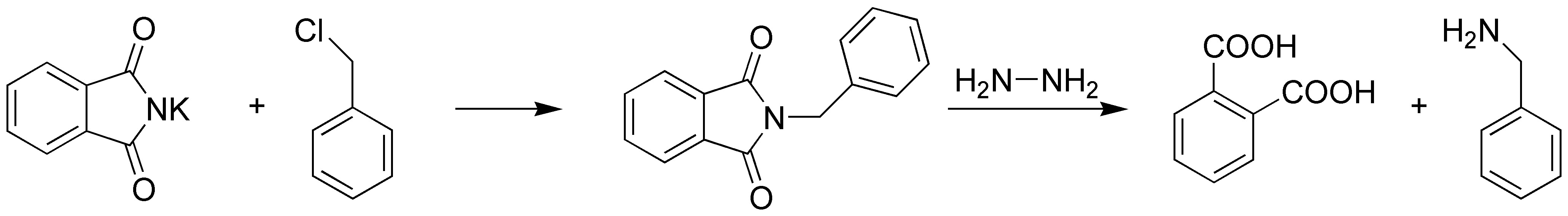
Solution 3:
These are aliphatic amines and ammonia, the higher basicity can be justified due to the fact that amines having more alkyl group will better stabilize the positive charge, of the product formed in the following equilibrium:
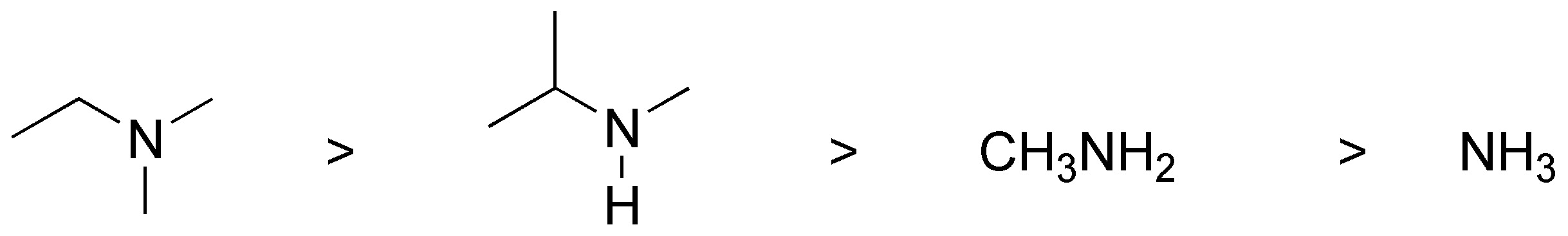
Amines with more alkyl groups will shift the equilibrium better to the right as the alkyl groups give up electrons by inductive effects. Therefore, the basicity increases according to the following order:
IV < II < I < III
Solution 4:
a) Basicity indicates the ability to accept a proton for this, in principle, the nitrogen atom must possess an unshared pair of electrons (which does not occur with compound V), on the other hand the higher the electron density on said N the higher the basicity (in aromatic amines the nitrogen electrons are partially delocalized in the ring and therefore they are less basic than aliphatic ones), therefore the order will be:
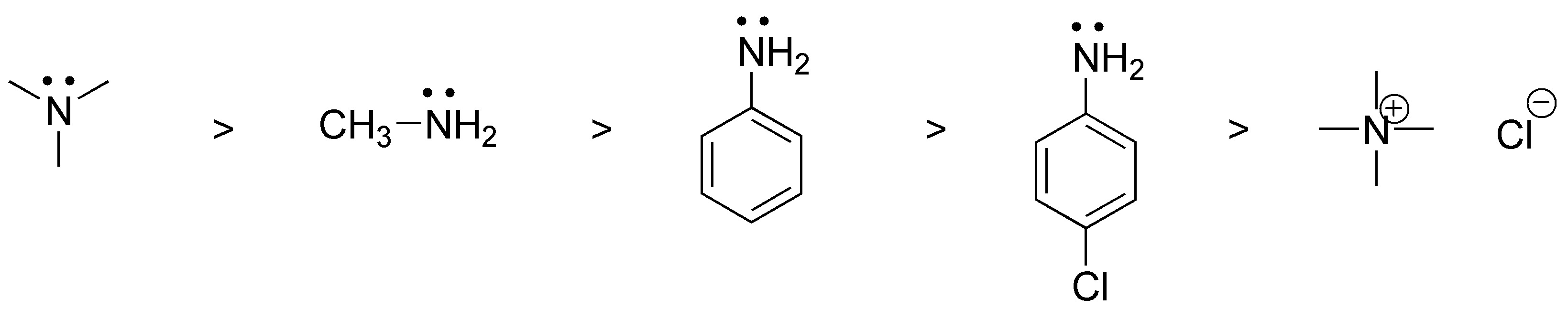
V < III < II < I < IV
b) Nucleophilicity indicates the ability to yield the electron pair to form a new C-N bond and apart from basicity steric factors also influence, therefore the order will be:
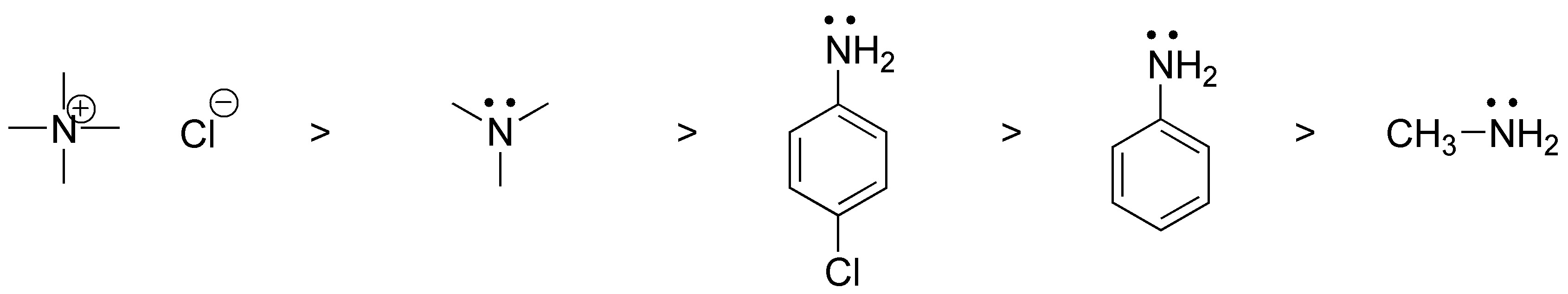
V < IV < III < II < I
Solution 5:
It will be carried out by acylation with benzoyl chloride:
Solution 6:
The reaction that occurs is the formation of a diazonium salt, which in the case of aliphatic amines, is unstable losing N2 and generating a primary carbocation that can react with a nucleophile or lose a proton giving an alkene. Since it is a primary carbocation it can undergo transpositions.
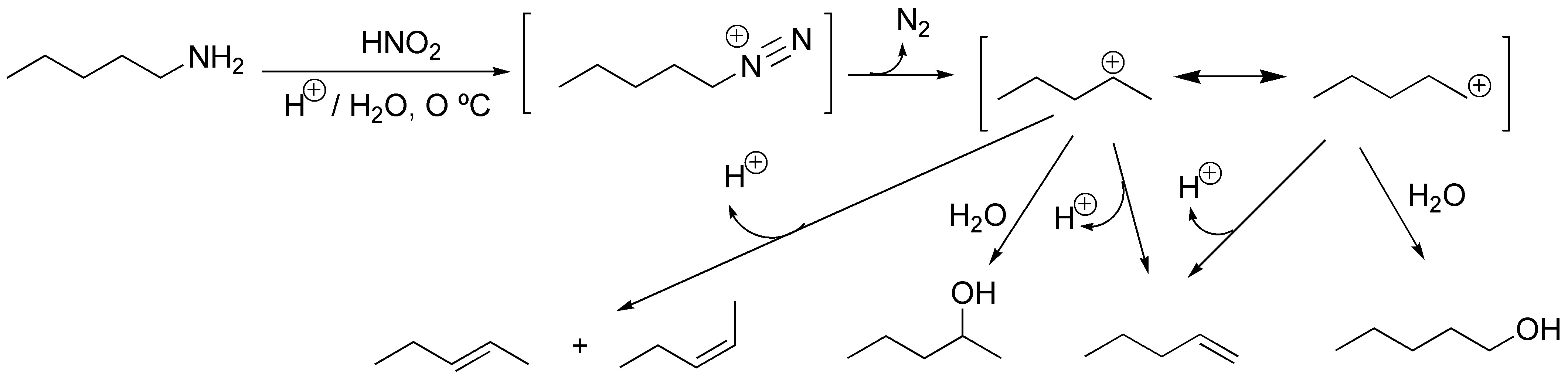
Solution 7:
The reaction corresponds to a Hofmann elimination. Since it requires only one mole of methyl iodide it must be a tertiary amine and since it produces but-1-ene it must have an n-butyl residue. It will therefore be butyldimethylamine.
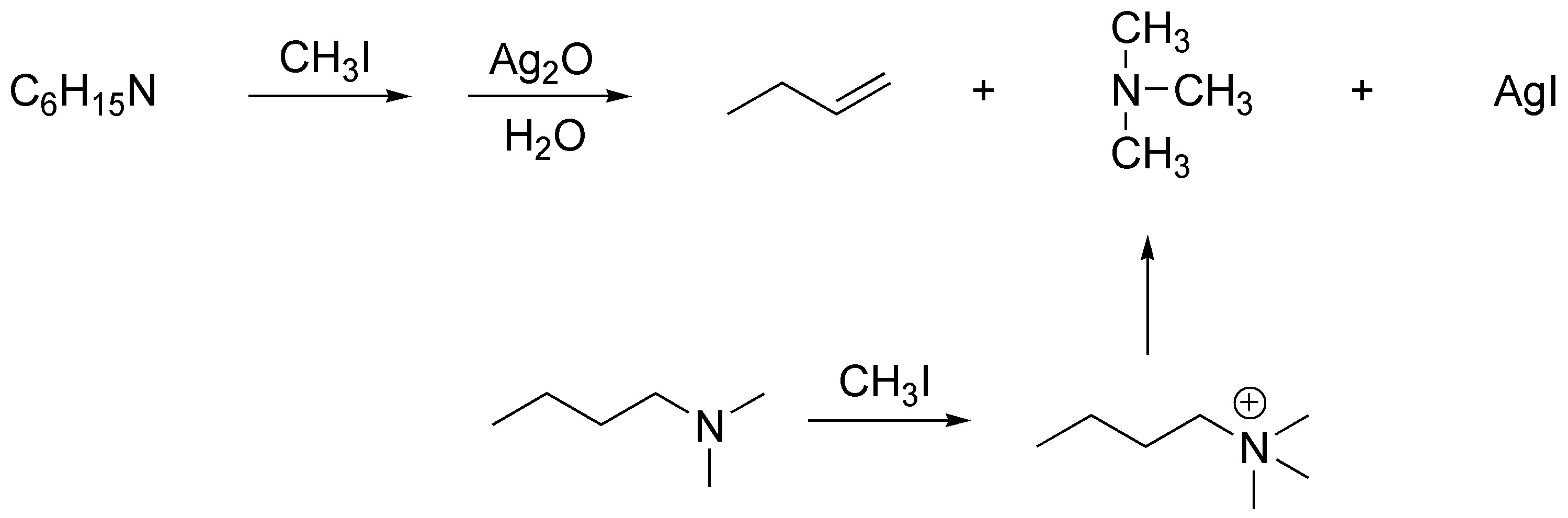
Solution 8:
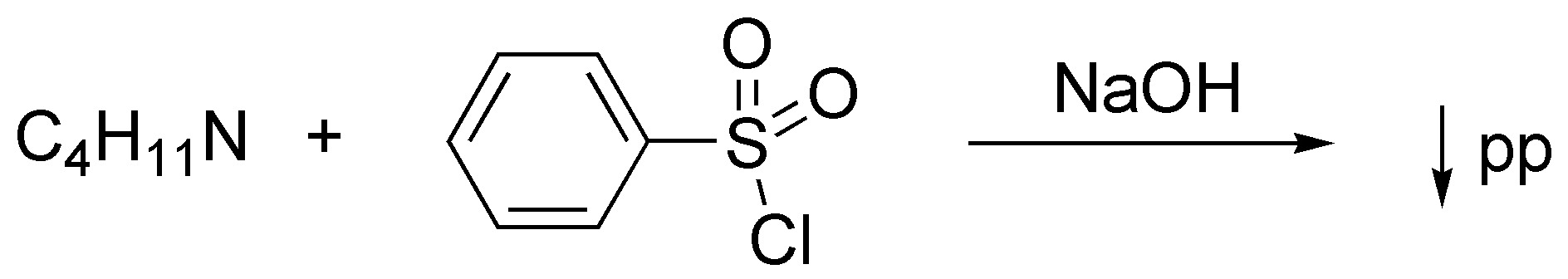
Corresponds to a Hinsberg test. Since the result of the same is the formation of an insoluble precipitate in basic medium the starting amine will be a secondary amine and therefore the solutions can be: diethylamine (I), methylpropylamine (II) or isopropylmethylamine (III).
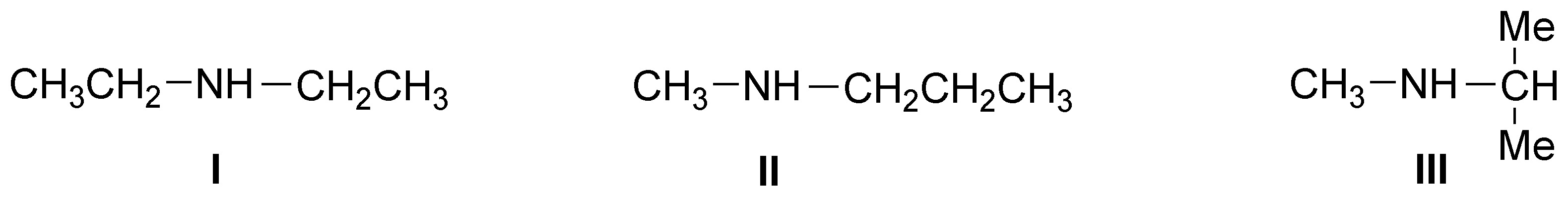
Solution 9:
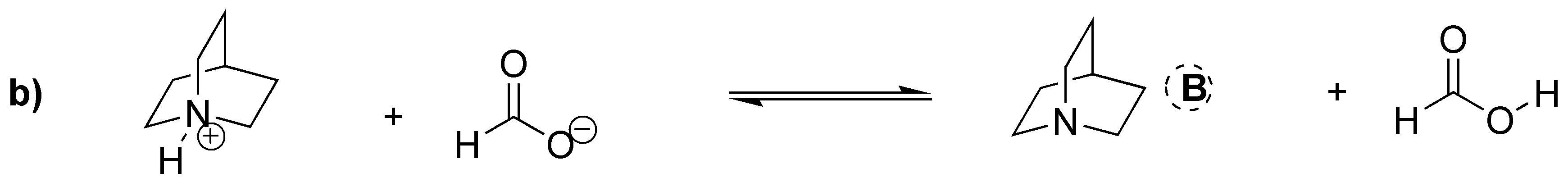
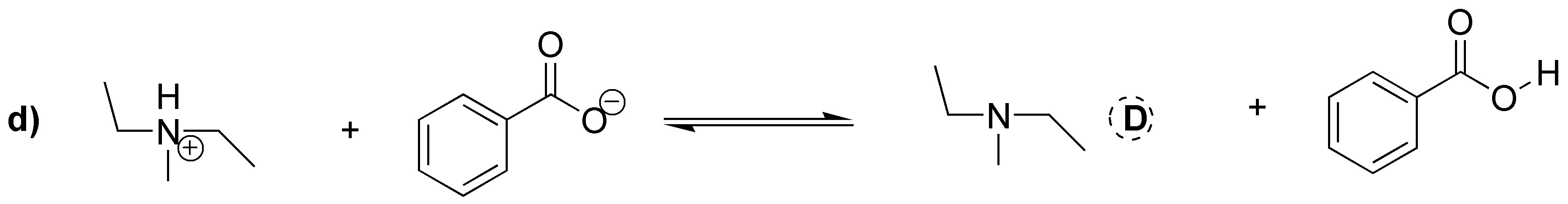
Acid-base equilibria between amines and carboxylic acids proceed by the transfer of a proton from the acid to the base. In the case of a) it is clear that it must be the protonated amine. In the case of b) however what we have is the carboxylate ion which acts as the conjugate base of the corresponding carboxylic acid and accepts the proton from the quaternary ammonium cation. In c) the primary amine is protonated and in d) the same thing happens as in b), that the ammonium cation acts as an acid yielding a proton to the carboxylate ion to give ammonia.

Solution 10:
Secondary, tertiary amines are obtained as intermediates which again react with methyl iodide to finally give the quaternary ammonium salt.
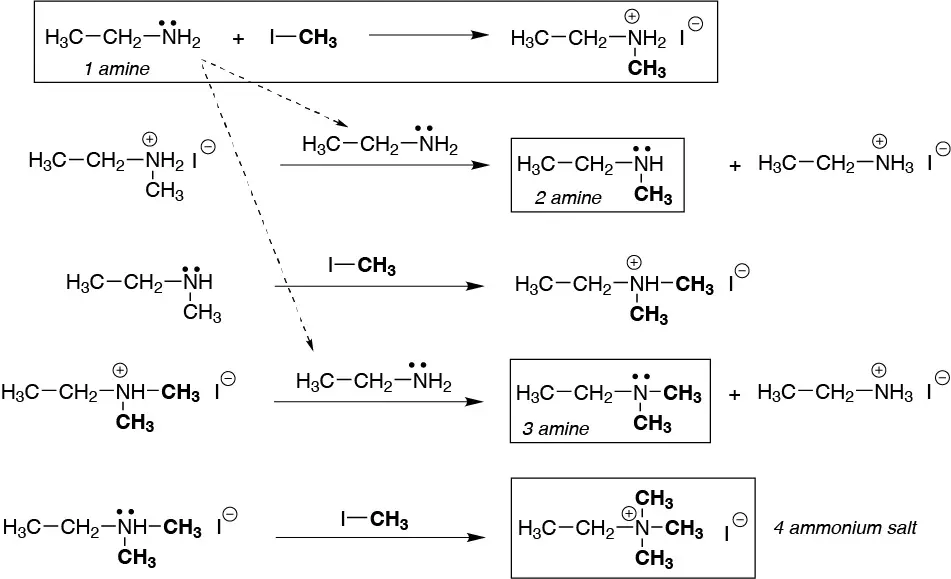
Solution 11:
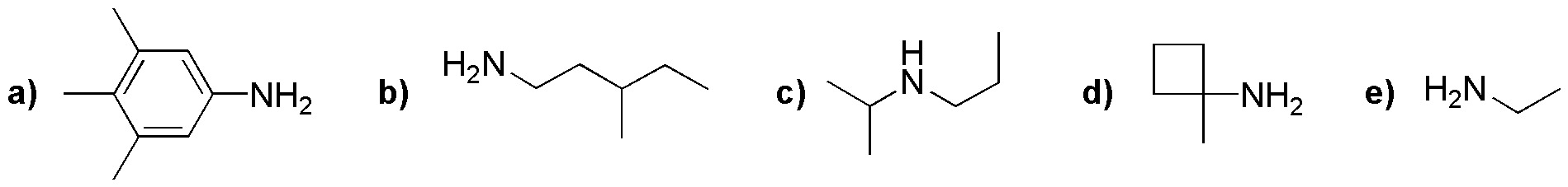
Gabriel synthesis generates primary amines, as they are obtained from the N-substituted phthalamide. Moreover, the first step of the Gabriel synthesis proceeds via an SN2 mechanism, which is favored with primary alkyl halides (little sterically hindered). Secondary amines such as c) are not obtained. Therefore, b) and e) are primary amines that could be obtained by this process. However, primary amines such as d) cannot be synthesized by this method since they are very sterically hindered. On the other hand, arylamines such as a) are ruled out, because in the corresponding starting halogenated derivative the SN2 reaction does not occur at the carbon of the aromatic ring.
Solution 12:
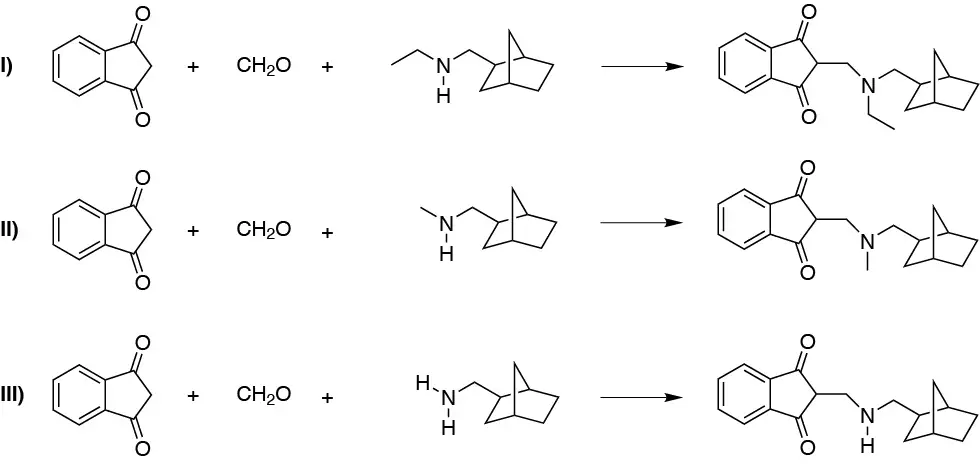
To carry out the Mannich reaction we need three reagents, a primary or secondary amine, a non-enolizable aldehyde and finally an enolizable carbonyl compound. Therefore we can rule out a priori, b), d), e), g), h), i), k), r) and q) because they have only two reactants. In addition, we can also rule out f), j), n) and o) because the amines are tertiary and these do not react in the Mannich reaction. Of the remaining a), c), l), m), and p), we discard a) because benzaldehyde is not enolizable and l) because although it meets the requirements of types of reactants, they do not give any of the products. Therefore, to obtain I it can only be done from m), for II from p) and to obtain compound III it would be done from c).
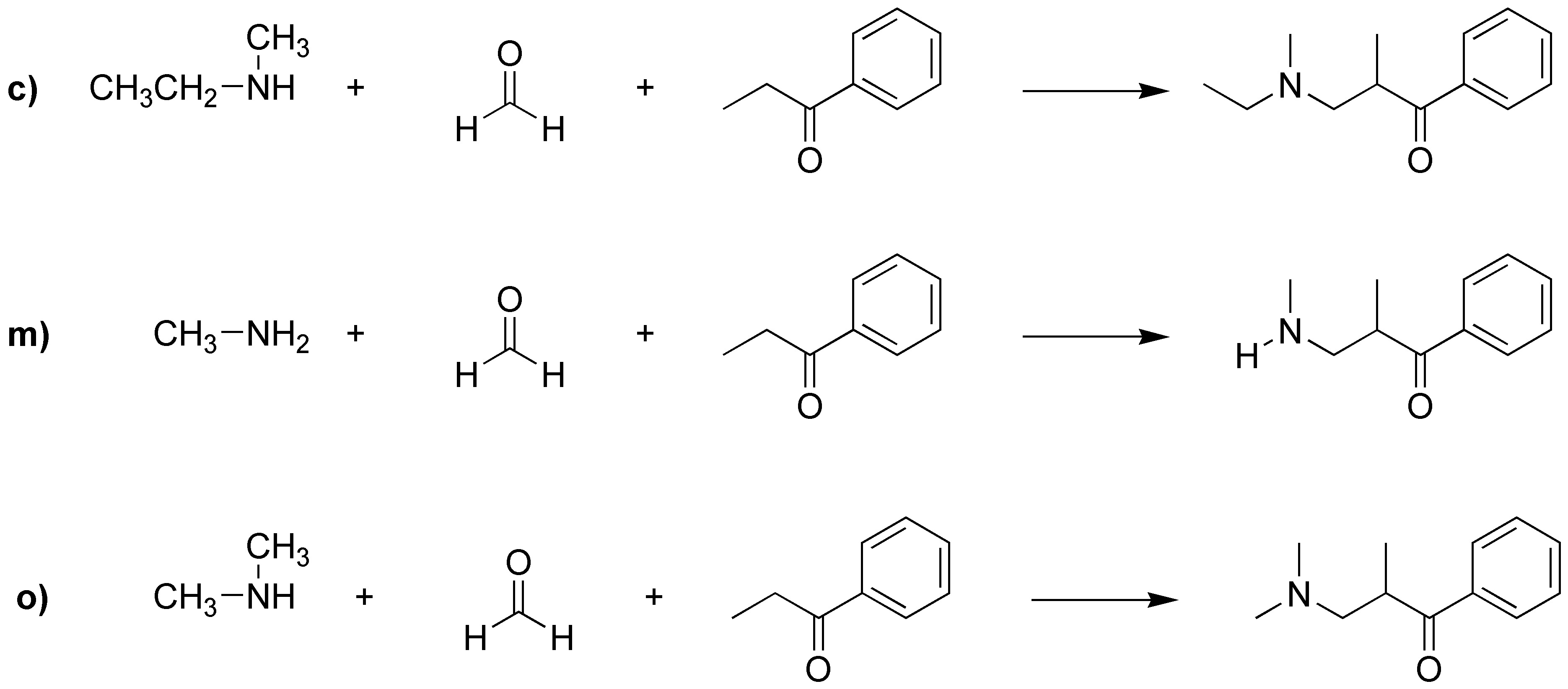
Solution 13:
The first reaction, with excess methyl iodide (MeI) the corresponding quaternary ammonium salts are produced. Subsequently, on heating in basic medium the less substituted alkenes are obtained as major products.



Solution 14:
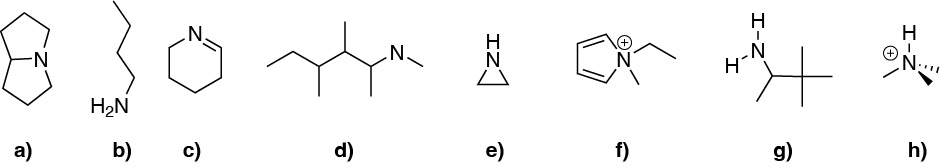
Compounds b) and g) are primary amines, d) and e) are secondary amines, a) and c) tertiary amines, and f) and h) quaternary amines.
Solution 15:


Solution 16:
The least substituted olefin is produced (Hofmann’s rule for eliminations).
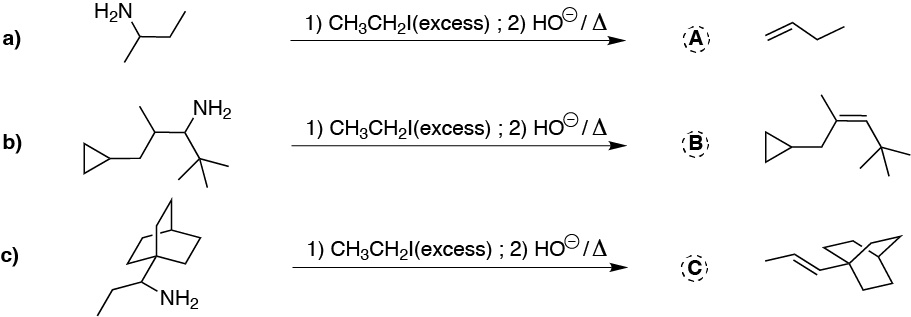
Solution 17:
Compound I is obtained from reagent a, g and i; compound II from reagent a, k and g; and compound III from reagent a, k and l.
Solution 18:
A) It will be the product resulting from SN2. B) It corresponds to a primary amine. C) It is a primary alkyl halide.
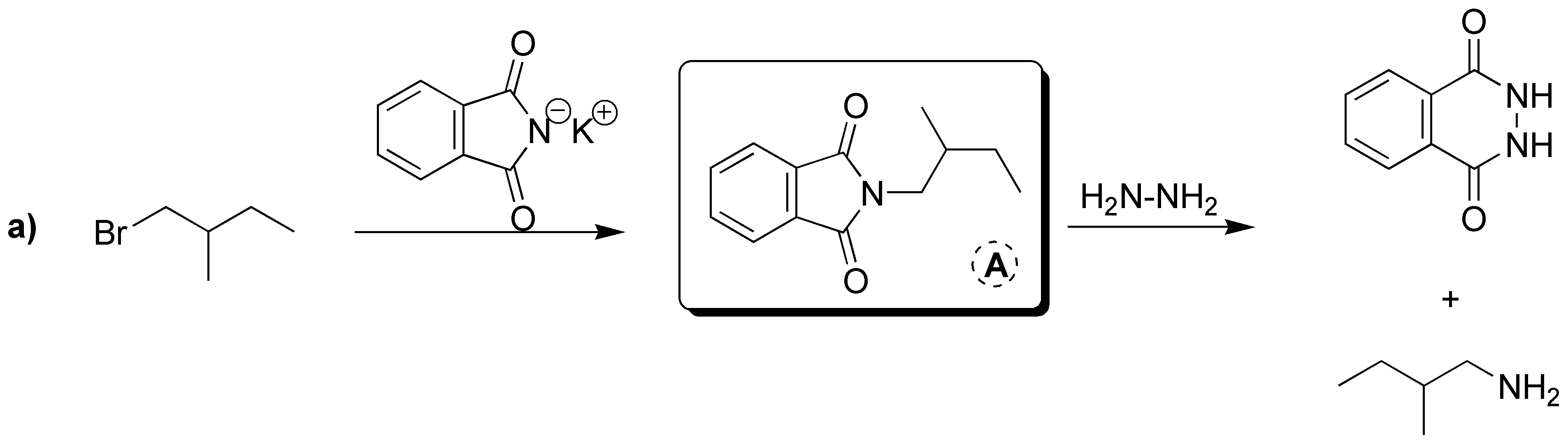
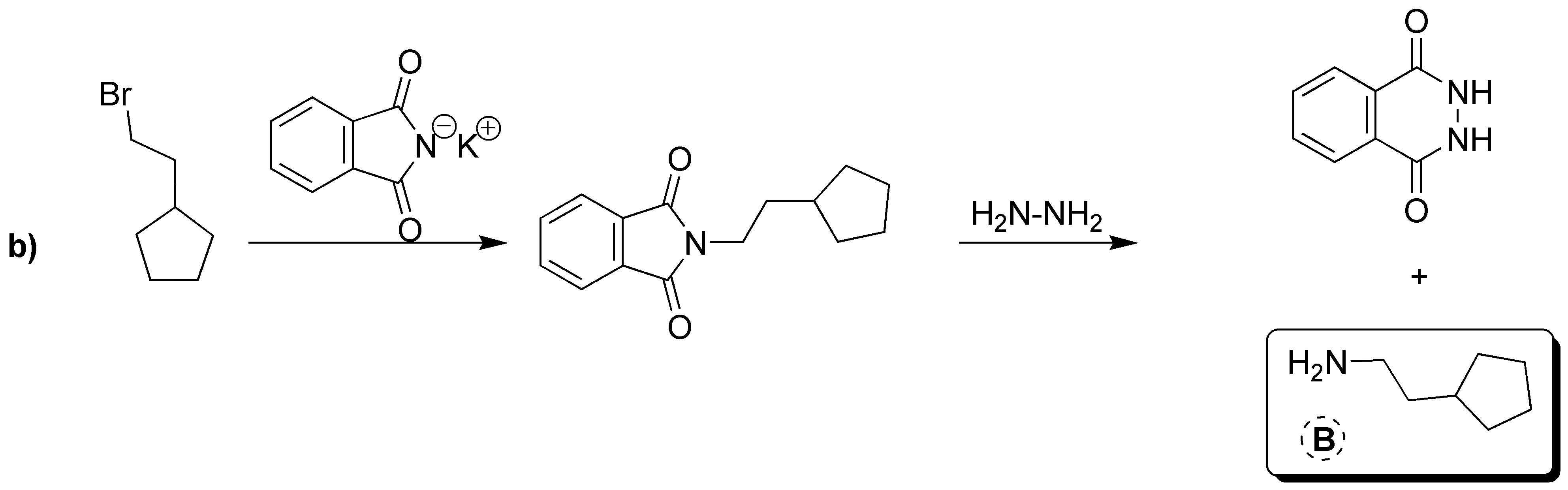
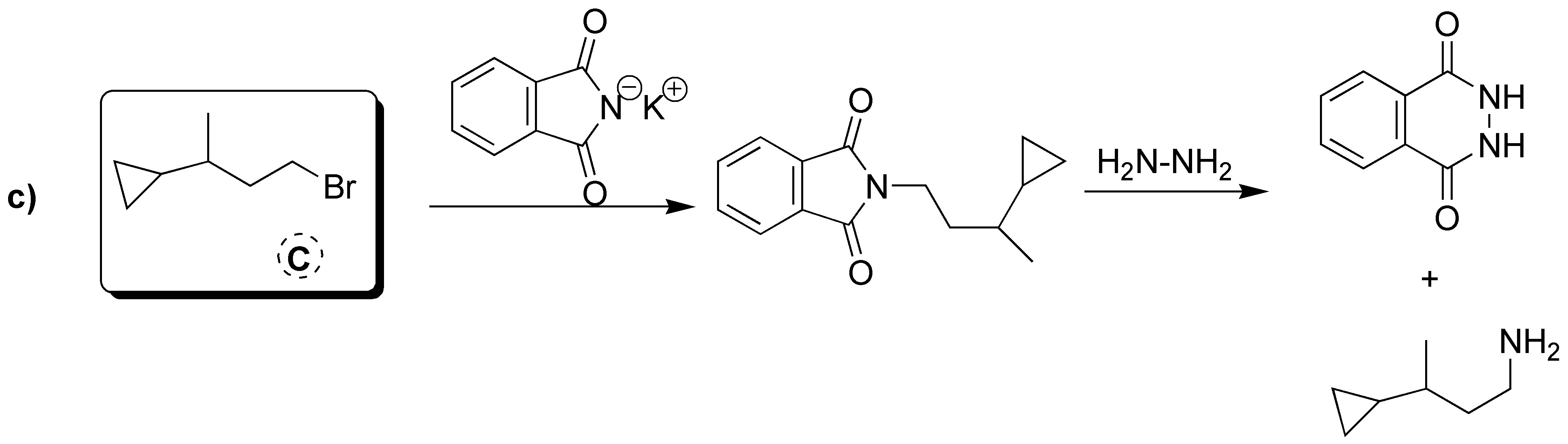
Solution 19:
The bromine atom that is less sterically hindered (less substituted) reacts earlier. Moreover, Br is a better leaving group than F, and the SN2 reaction proceeds on Br.


Solution 20:
Primary amines yield diazonium salts. Secondary nitrosoamines, tertiary aliphatic ones do not react. Tertiary aromatics undergo nitrosation reaction (SAE).
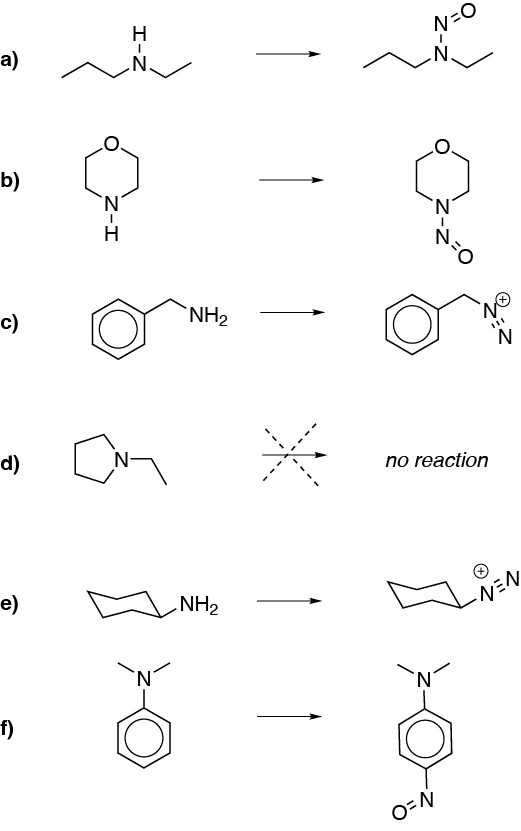
Solution 21:
- a) As they have the same number of carbon atoms and there is no direct acid to amino conversion, we would proceed to the conversion of the acid group to a haloalkane after reduction to alcohol and finally a SN2 would be performed.
- b) Having increased the chain by one carbon atom, we would proceed by means of a SN2 with cyanide and subsequent reduction of the cyano group.
- c) The easiest conversion amino to alcohol is through the diazonium salt.
- d) It is a Hofmann elimination.
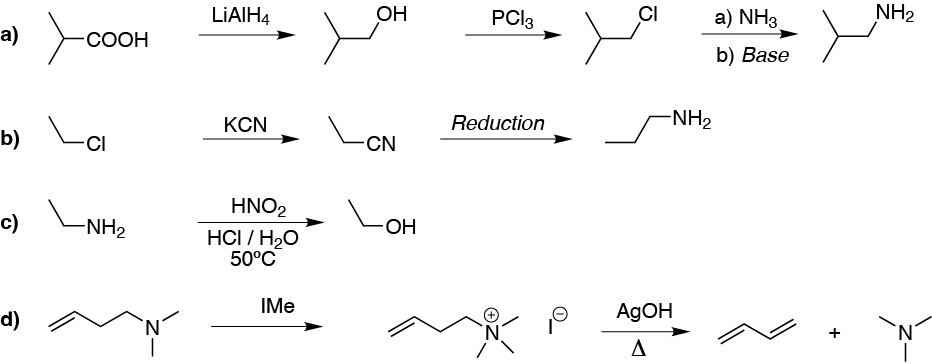
Solution 22:
Hofmann elimination yields the least substituted alkene:
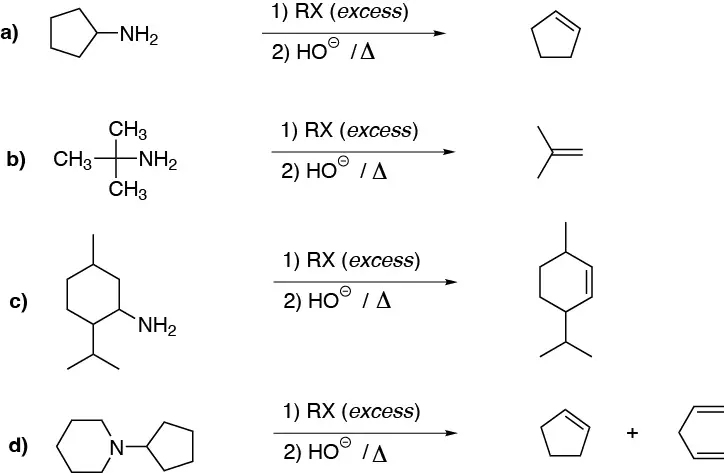
Solution 23:
The potassium phthalimide acts as a nucleophile against the benzyl halide.